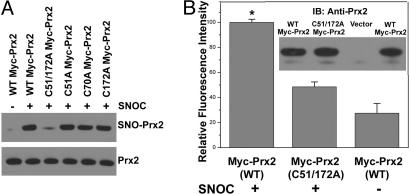
Fig. 2.
S-nitrosylation of Prx2 Cys-51 and Cys-172. (A) HEK-293T cell lysates transduced with WT or C-to-A mutant myc-Prx2 protein were exposed to 200 μM SNOC or control for 30 min. (Upper gel) SNO-Prx2 was detected by the biotin-switch assay by using anti-myc antibody. (Lower gel) Total protein expression level was determined by Western blotting using anti-myc antibody. Mutation of critical cysteine thiol groups on Prx2 (C51A/C172A) prevented S-nitrosylation by SNOC. (B) HEK-293T cells overexpressing WT or mutant (C51A/C172A) myc-Prx2 were exposed to 200 μM SNOC or control for 30 min, and myc-tagged Prx2 was immunoprecipitated by using an anti-Myc agarose conjugate. Immunocomplexes were subjected to the DAN assay. Fluorescence intensity was normalized to the value obtained for WT Myc-Prx2. *, P < 0.01 (n = 3). (Inset) Immunoblotting was used to ensure equal amounts of Prx2 in the immunocomplex.