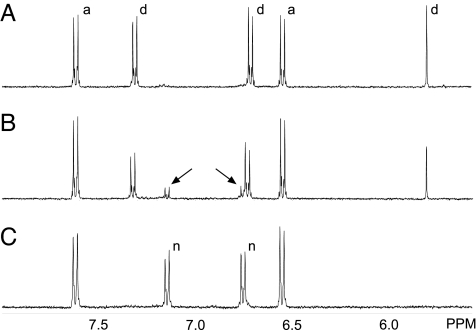
Fig. 7.
In vitro-breakdown of dhurrin as monitored by 1H-NMR. (A) Spectrum recorded 40 min after dissolving dhurrin (4 mM) in 100 mM KDCO3 (pH 8.3). Approximately half the amount of dhurrin is converted into 4-hydroxybenzaldehyde. (B) Spectrum recorded 20 min after addition of DTT (2 mM). 4-Hydroxyphenylacetonitrile formation is apparent (arrows). (C) As in B, except that the spectrum was recorded after 5 h. All dhurrin has now been converted into 4-hydroxybenzaldehyde and 4-hydroxyphenylacetonitrile. Signals in the 5.5- to 8.0-ppm region are shown. Signals assigned to dhurrin (d), 4-hydroxybenzaldehyde (a), and 4-hydroxyphenylacetonitrile (n) are labeled.