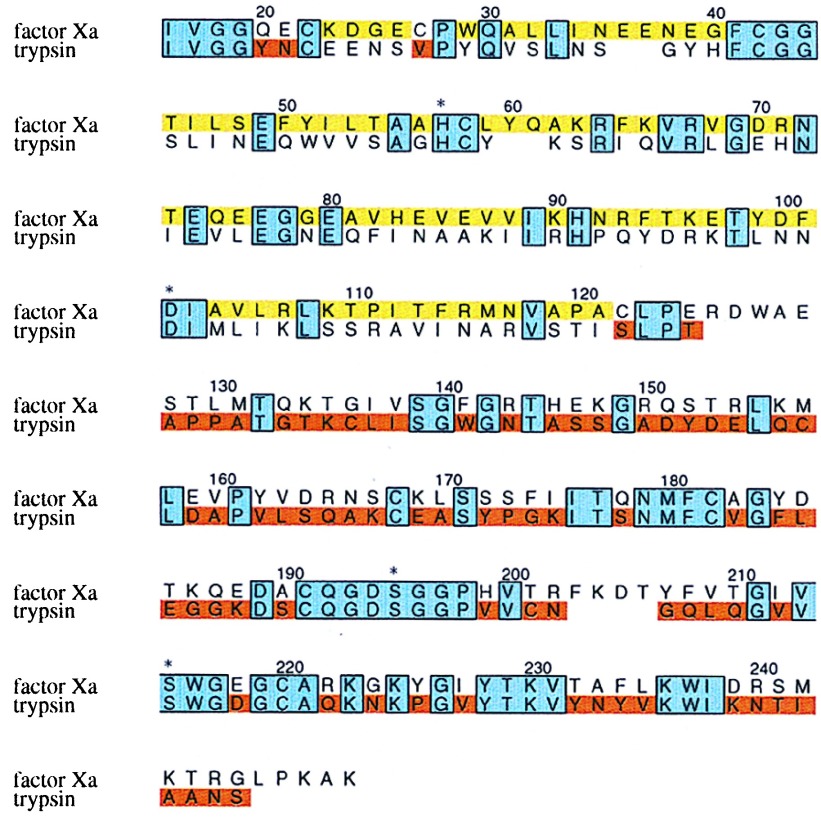
Figure 1.
Primary structure of the fXa/trypsin hybrid. The primary structure of rfXYa is shown in an amino acid sequence alignment (single letter code) of the catalytic domain of human coagulation factor Xa and human trypsin 1. Conserved residues (38% sequence identity) are boxed and shaded blue. Segments taken from fXa are colored yellow; those from trypsin are colored red. Three residues at the N terminus (Y20,E21 and V27) were taken from trypsin (red) to account for a different disulfide bridge in fXa (C22-C27) and trypsin (C22-C157). The trypsin disulfide bridge C22-C157 was used. Both subdomains contribute to the catalytic tetrad (denoted by asterisk).