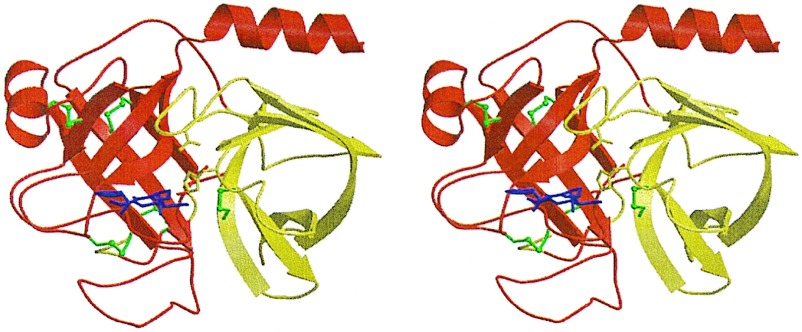
Figure 2.
Crystal structure of the fXa/trypsin hybrid. Ribbon plot of the crystal structure of fXYa. The N-terminal subdomain is shown in red, and the C-terminal subdomain is shown in yellow. Both subdomains adopt a β-barrel fold and assemble asymmetrically to generate the fold typical of the chymotrypsin family. Disulfide bridges are depicted in green (the N-terminal bridge discussed in the text is located behind the C-terminal barrel). The D-Phe-Pro-Arg inhibitor is shown with magenta sticks. It is bound to the active site, which is formed at the subdomain interface. The catalytic triad residue side chains are displayed explicitly as sticks.