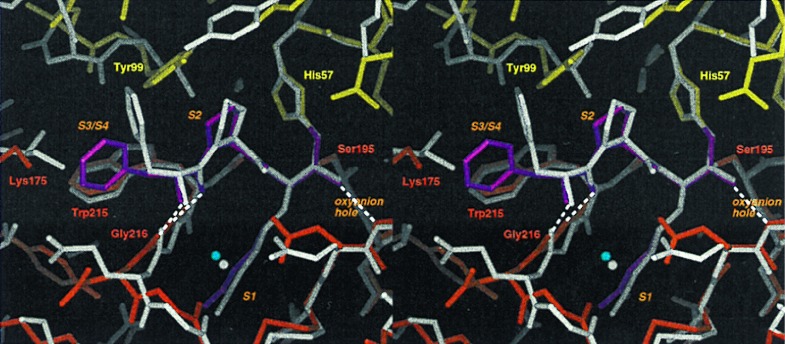
Figure 5.
Inhibitor binding. The active site of PPACK-rfXYa (colored sticks using the code of Fig. 2) superimposed with the active site of PPACK-thrombin (grey sticks; PDB ID code 1ppb). PPACK binds in a substrate-like binding mode: P1-arginine extends into the S1-pocket; the backbone forms a β-sheet with the enzyme backbone at positions 214–216; and P2-proline occupies the S2-pocket. Characteristic hydrogen bonds of the transition state–serine proteinase interaction are formed (dashed lines). The binding mode is identical to that observed in PPACK thrombin except for the orientation of the P3 sidechain: in thrombin, the phenyl moiety of P3 fits into the S3/S4 site whereas in rfXYa it is rotated toward the bulk solvent.