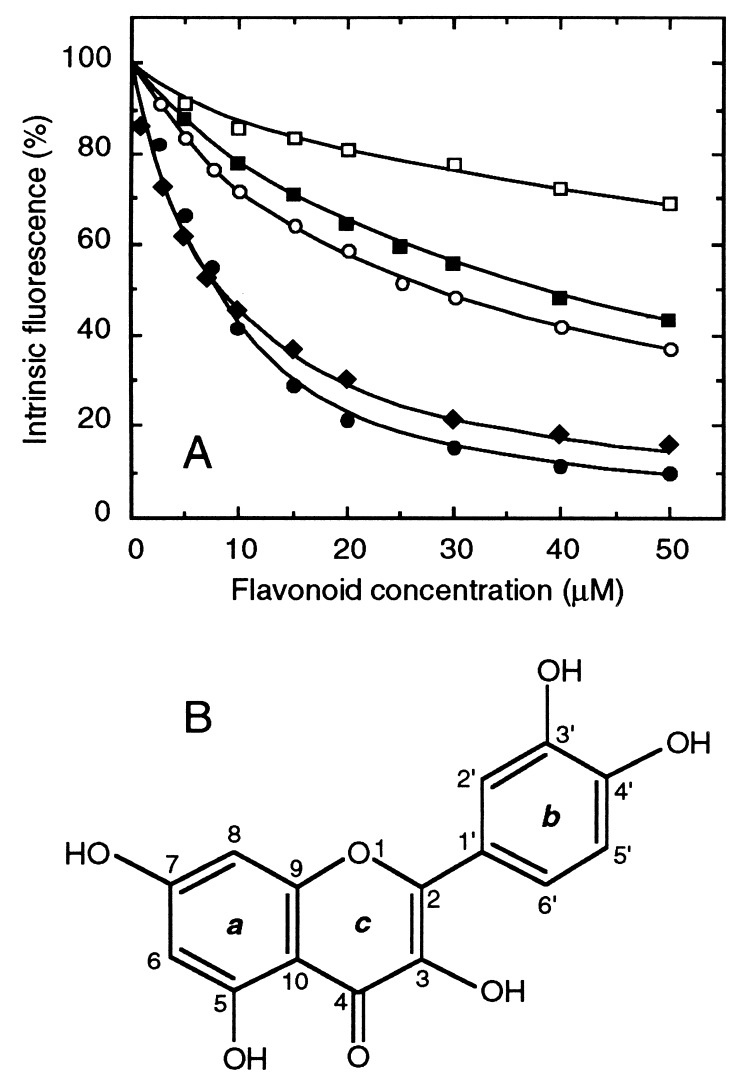
Figure 2.
Differential quenching of H6-NBD2 intrinsic fluorescence by quercetin and other flavonoid modulators. (A) The interaction between different classes of flavonoids and 1.1 μM H6-NBD2 was studied under the same conditions as described for MANT-ATP in Fig. 1C. Increasing concentrations of either quercetin [3,5,7,3′,4′-pentahydroxyflavone (•), whose chemical structure is shown in B], apigenin [5,7,4′-trihydroxyflavone (⧫)], naringenin [2,3-dihydroapigenin (○)], genistein [isoapigenin (■)], or rutin [3-O-glucorhamnosyl quercetin (□)] were used as dimethyl sulfoxide solutions, up to a 2% (vol/vol) final concentration.