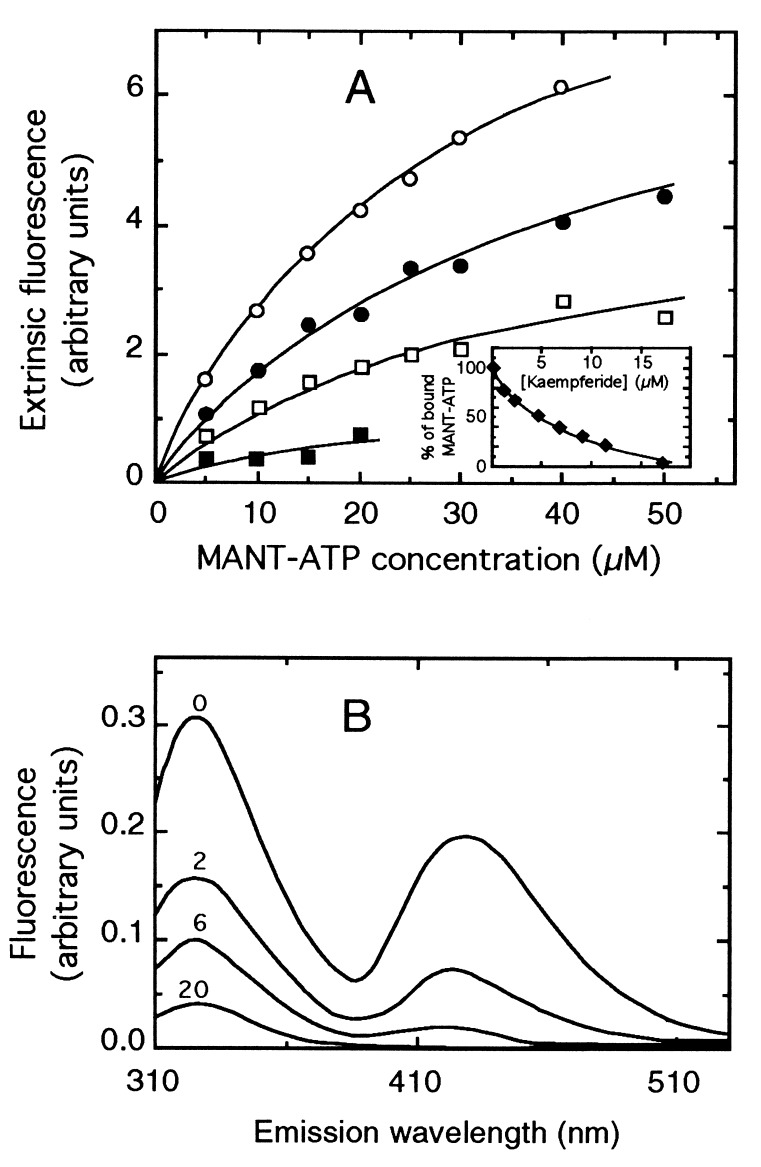
Figure 3.
Complete antagonism of kaempferide against MANT-ATP binding to H6-NBD2. (A) The H6-NBD2 domain (6 μM) was preincubated without (○) or with 3 (•), 6 (□), or 10 (■) μM kaempferide, and then assayed for MANT-ATP binding by the increase in extrinsic fluorescence between 420 and 460 nm upon excitation at 350 nm. (Inset) Decrease in extrinsic fluorescence related to displacement of bound MANT-ATP, upon incubation of 3.2 μM H6-NBD2 with 20 μM MANT-ATP, by addition of increasing kaempferide concentrations. (B) The residual fluorescence resonance-energy transfer, produced upon incubation of 2.1 μM H6-NBD2 with 20 μM MANT-ATP and excitation at 295 nm under conditions specified in Fig. 2A, was measured after addition of increasing kaempferide concentrations, from 0 to 20 μM, as indicated on each trace.