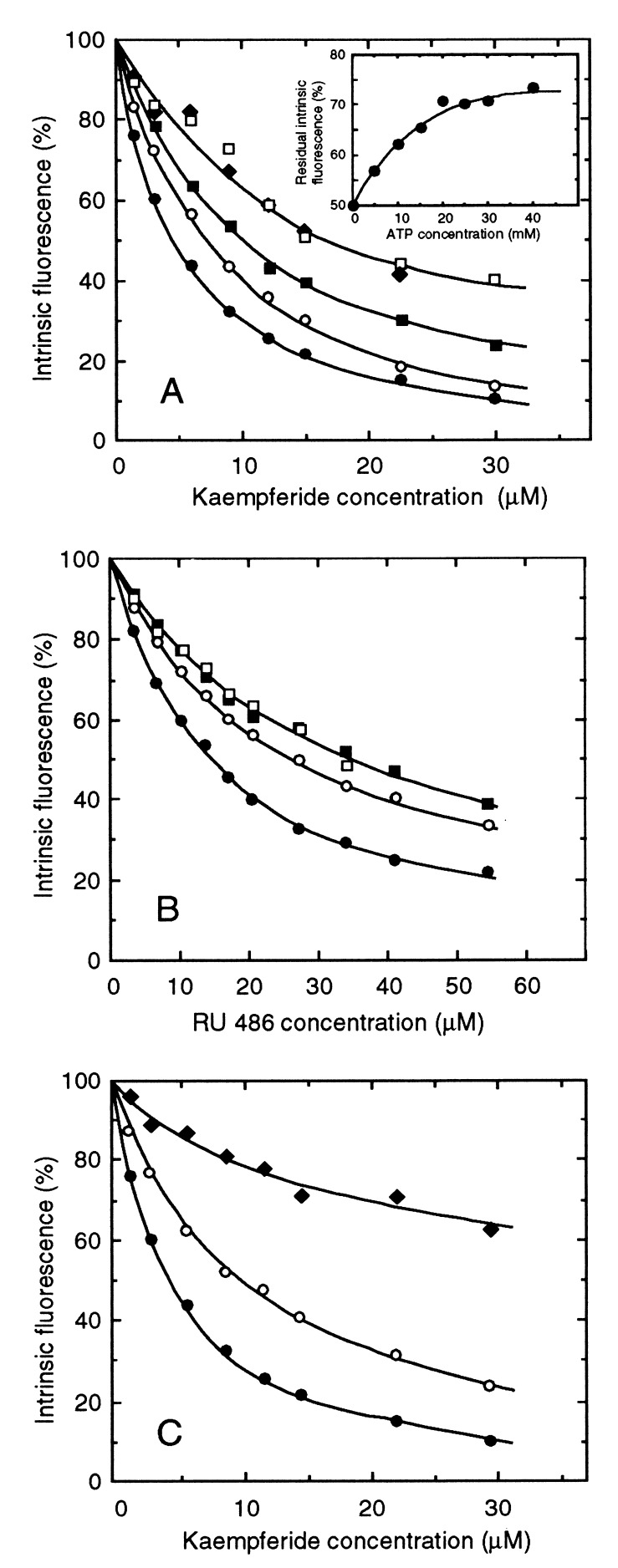
Figure 4.
Partly antagonistic bindings of RU 486, kaempferide, and ATP. (A) The H6-NBD2 domain was preincubated at 1.1 μM in the absence (•) or presence of ATP at 10 (○), 20 (■), 30 (□), or 40 (⧫) mM and assayed for quenching of intrinsic fluorescence upon addition of increasing kaempferide concentrations. (Inset) The 50% quenching produced by incubation of H6-NBD2 with 4.5 μM kaempferide was partly released upon addition of increasing ATP concentrations, up to 40 mM. (B) Partial prevention against RU 486 binding (•) by preincubating H6-NBD2 with 5 (○), 10 (■), or 20 (□) μM kaempferide. (C) Partial prevention against kaempferide binding (•) by preincubation with 24 μM RU 486 alone (○) and additional effect by preincubation with both 24 μM RU 486 and 20 mM ATP (⧫).