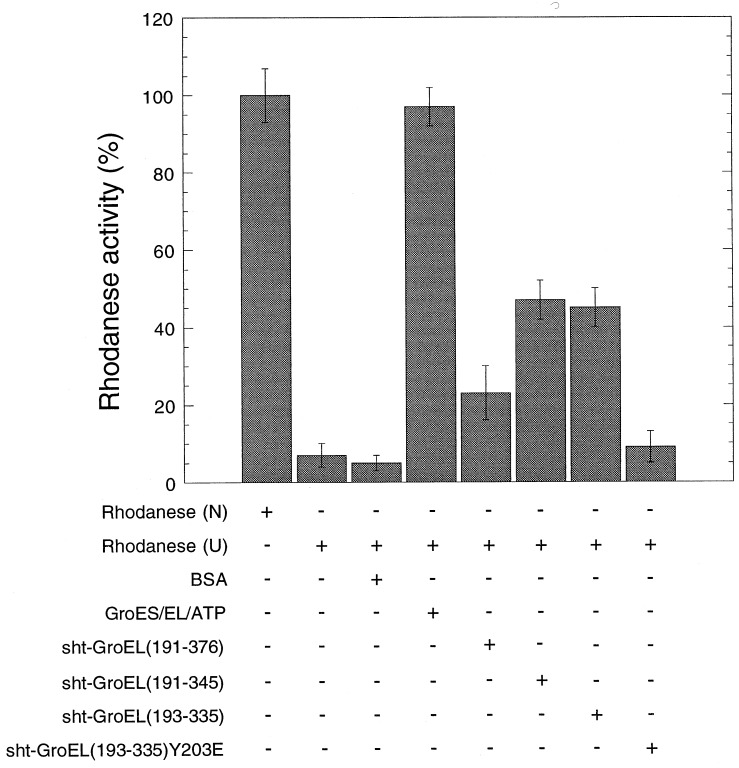
Figure 3.
In vitro refolding of rhodanese in the presence of GroEL minichaperones. Relative enzymatic activity of rhodanese (0.1 μM) after refolding in the presence (+) or absence (−) of GroEL (2.5 μM monomer), GroES (2.5 μM monomer), ATP (2 mM), sht-GroEL191–376 (2.5 μM), sht-GroEL191–345 (2.5 μM), sht-GroEL193–335 wild-type and mutant Y203E (2.5 μM), or BSA (45 μg/ml), from 8 M urea (U). The yield of refolding activity was measured after 15 min at 25°C. One-hundred percent activity was obtained with native rhodanese (N). Standard error bars are shown.