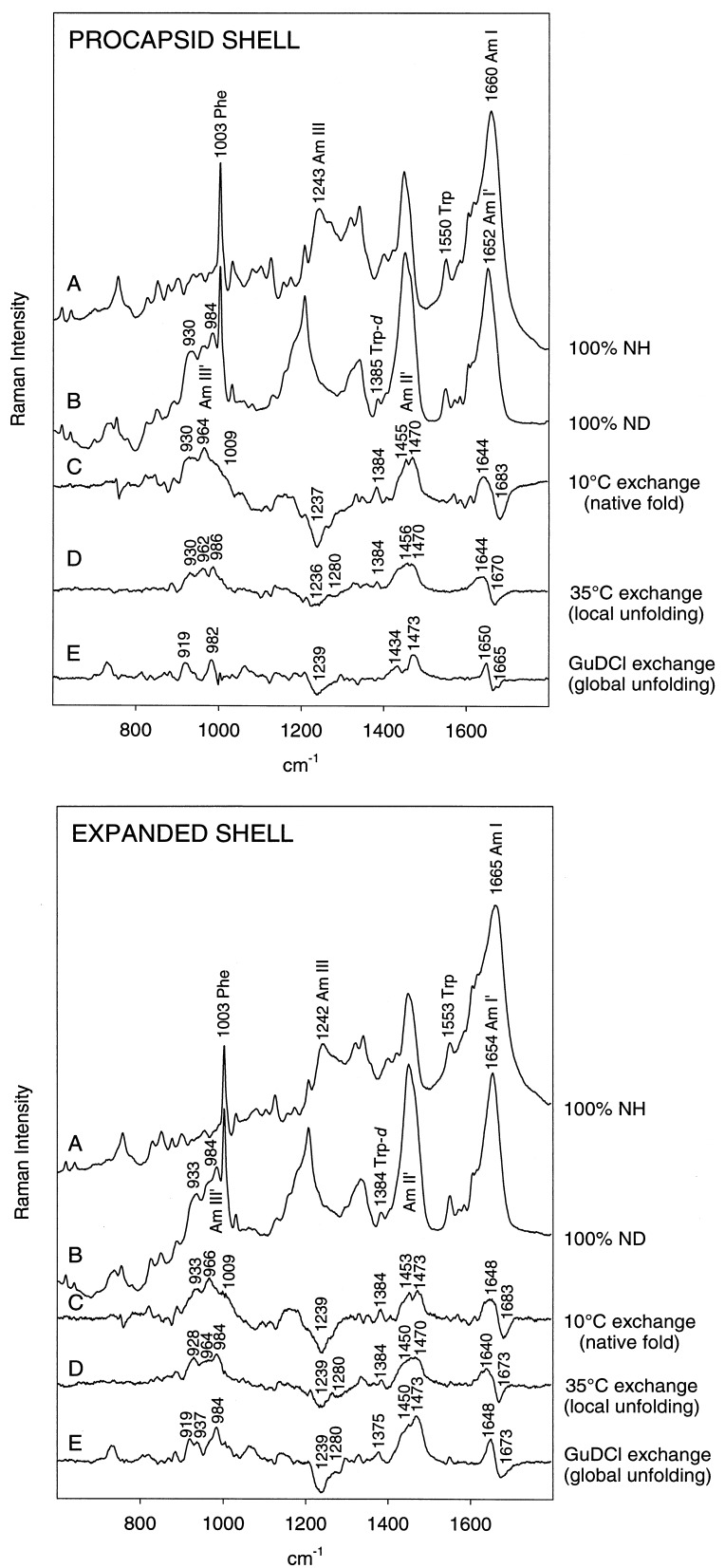
Figure 1.
Raman monitoring of deuteration of peptide NH groups of procapsid (Upper) and expanded shells (Lower). In each panel: (A) H2O solution spectrum. (B) D2O solution spectrum of fully deuterated particle, obtained by refolding and reassembly in native D2O buffer of a sample previously treated with 6 M GuDCl. (C) Difference spectrum between A and particles exposed to D2O solution for 3 h at 10°C (signature of peptides exchanging from the native state). (D) Difference spectrum between particles exposed to D2O for 600 h at 35°C and particles exposed to D2O for 3 h at 10°C (signature of peptides exchanging via local unfolding). (E) Difference spectrum between B and particles exposed to D2O for 600 h at 35°C (signature of peptides in the exchange-protected core). Spectra were obtained from samples at 70 mg/ml concentration in 10 mM Tris buffer (pH 7.4) and 10°C.