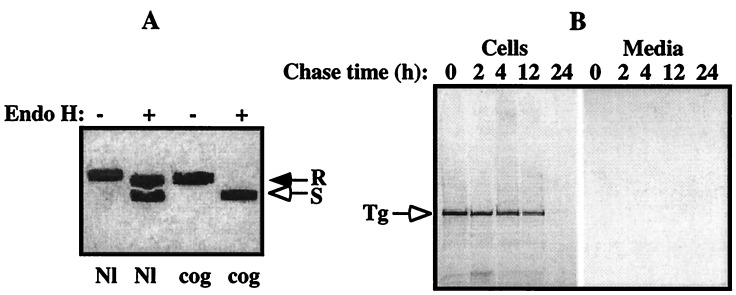
Figure 4.
Cog Tg is retained in the ER and degraded intracellularly. COS-7 cells, transfected with nonmutant or cog Tg cDNAs as in Fig. 3, were pulse-labeled with [35S]methionine/cysteine, chased for various times, and the medium collected and cells lysed as described. (A) Equal aliquots of cell lysates and chase medium were combined and immunoprecipitated with polyclonal antiserum against mouse Tg, prior to digestion with endo H (+) or mock digestion (−). As shown at 6 h of chase, ≥60% of normal Tg had passed the medial Golgi as measured by acquisition of resistance to endo H digestion (R) with secretion into the medium (not shown), whereas cog Tg remained entirely sensitive (S) to endo H digestion (open arrow), indicating that the mutant Tg protein never reached the Golgi for complex carbohydrate addition. (B) Equal aliquots of lysates of cells transfected with the cog Tg cDNA, and the corresponding chase medium samples, were immunoprecipitated with antisera against Tg and analyzed by SDS/PAGE and fluorography. Although ≈40% of cog Tg is slowly degraded over the first 12 h, during the second 12-h chase, the remaining newly synthesized cog Tg was more rapidly degraded with an undetectable fraction appearing in the secreted media, consistent with biphasic degradation kinetics (39).