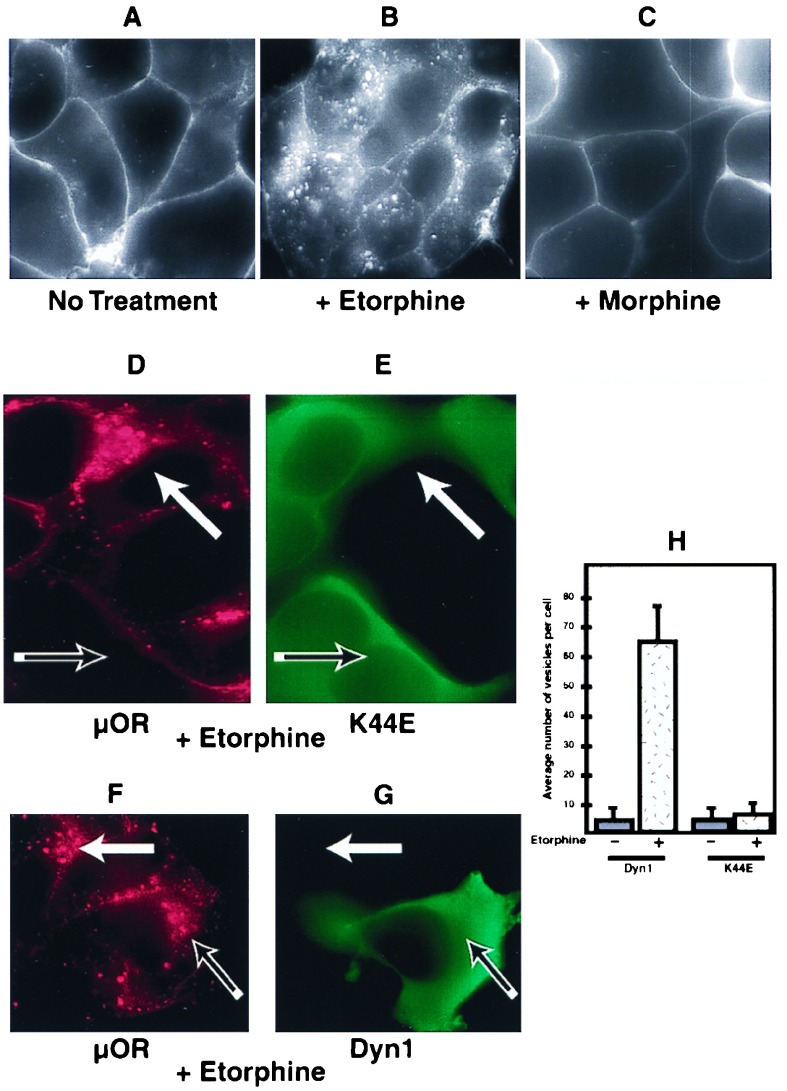
Figure 1.
Mu opioid receptors were endocytosed in a Dyn-dependent manner. HEK293 cells expressing FLAG-epitope-tagged μ opioid receptors (μOR) were stained as described in Materials and Methods. (A) Receptors remained predominantly in the plasma membrane in the absence of agonist stimulation. (B) Cells were treated with 5 μM etorphine for 30 min at 37°C and stained. In the presence of etorphine, receptors were endocytosed as indicated by redistribution of antibody-labeled receptors from the plasma membrane to numerous endocytic vesicles visualized throughout the cytoplasm. (C) Cells were treated with 5 μM morphine for 30 min at 37°C and stained. In the presence of morphine, receptors remained in the plasma membrane. (D and E) HEK293 cells expressing the FLAG-tagged μ opioid receptor were transfected transiently with an HA-tagged dominant negative Dyn, K44E. Cells were then treated with 5 μM etorphine and stained for both receptor in red (D) and Dyn in green (E). Cells expressing K44E Dyn failed to endocytose the receptor (D and E, open arrows), whereas adjacent cells not expressing K44E Dyn did endocytose receptor (D and E, closed arrows). (F and G) Cells transiently transfected with HA-tagged wild-type Dyn also were treated and stained. HA-tagged wild-type Dyn did not affect receptor endocytosis, as cells expressing (F and G, open arrows) and not expressing (F and G, closed arrows) Dyn-endocytosed receptors. (H) Slides stained as above were coded, and receptor-containing vesicles were counted from cells expressing both wild-type and mutant Dyn as well as cells not expressing any additional Dyn.