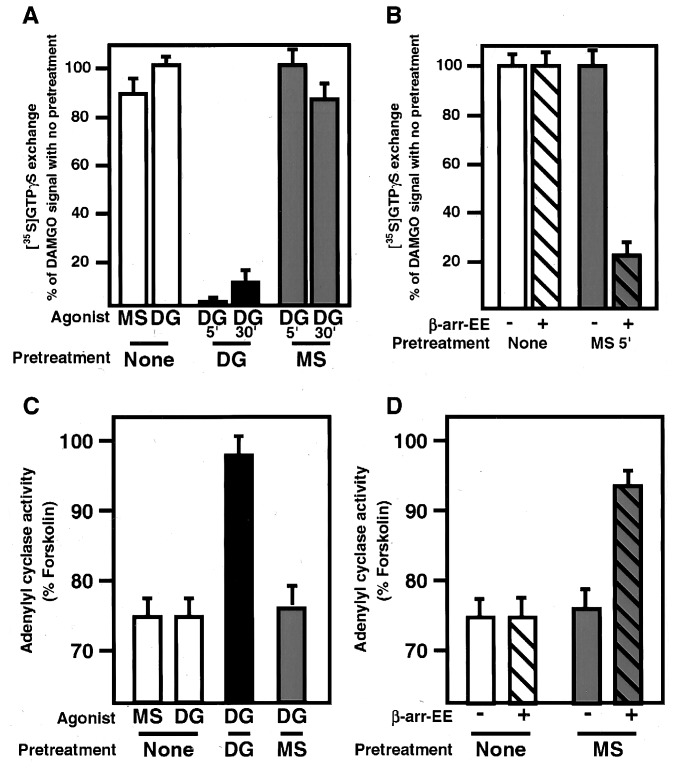
Figure 3.
Morphine stimulated GTP exchange but failed to promote uncoupling of receptor from G protein and failed to desensitize adenylyl cyclase activity. (A) Cells expressing μ opioid receptor were pretreated for either 5 or 30 min with DAMGO (DG) or morphine (MS) or left untreated and residual agonist washed from cells. Membranes were prepared from these cells and the ability of the μ receptors in these membranes to activate GTP exchange on G proteins in vitro was measured. Receptors from cells that were not pretreated with agonist stimulated GTP exchange efficiently with both morphine and DAMGO (open bars). Receptors pretreated with DAMGO for either 5 or 30 min were very inefficient at promoting GTP exchange upon DAMGO stimulation (black bars), indicating that the μ opioid receptors in these cells had become uncoupled from their G proteins during the DAMGO pretreatment. Receptors pretreated with morphine were still as effective at stimulating GTP exchange upon DAMGO stimulation (shaded bars) indicating that pretreatment with morphine failed to uncouple the receptors from their G proteins. (B) Cells expressing both μ opioid receptor and EE-tagged β-arrestin were pretreated with morphine or left untreated and membranes prepared as above. Receptors from cells overexpressing arrestin (hatched bars) pretreated with morphine were significantly impaired in their ability to stimulate GTP exchange, differing markedly from cells expressing endogenous levels of arrestin (solid bars), demonstrating that overexpression of arrestin facilitated functional uncoupling of morphine-bound opioid receptors. These assays were done in triplicate three times with comparable results. (C) Cells expressing μ opioid receptor were pretreated for 5 min with DAMGO (DG) or morphine (MS) or left untreated, and residual agonist washed from cells. Membranes were prepared from these cells, and the ability of the μ receptors in these membranes to inhibit forskolin-stimulated adenylyl cyclase activity was measured. Receptors from cells that were not pretreated with agonist inhibited adenylyl cyclase efficiently with both morphine and DAMGO stimulation (white bars). Receptors from cells pretreated with DAMGO for 5 min were significantly impaired in their ability to inhibit adenylyl cyclase activity upon DAMGO stimulation (black bar). Receptors from cells pretreated with morphine were still as effective at inhibiting adenylyl cyclase upon DAMGO (shaded bar). (D) Cells expressing μ opioid receptor and overexpressing β-arrestin-EE were pretreated for 5 min with morphine (MS) or left untreated, and residual agonist washed from cells. Membranes were prepared and the ability of the μ receptors to inhibit forskolin-stimulated adenylyl cyclase activity was measured. Receptors from cells that were not pretreated with agonist inhibited adenylyl cyclase as efficiently as cells expressing endogenous arrestins. Cells overexpressing arrestin that were pretreated with morphine were significantly less efficient at inhibiting adenylyl cyclase than cells expressing endogenous levels of arrestins (cf. hatched and unhatched shaded bars). These assays were repeated twice in triplicate with similar results.