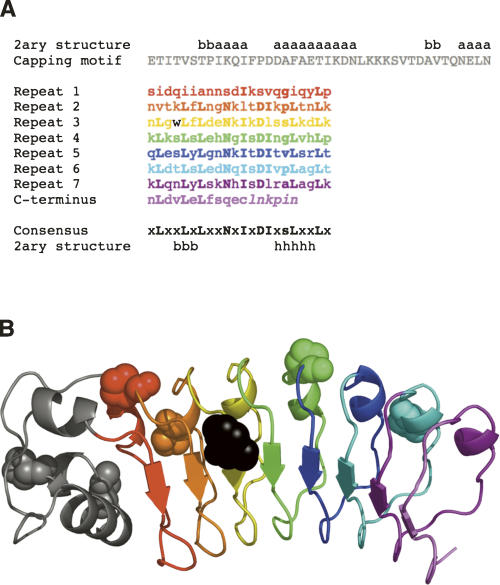
Figure 1.
Sequence and ribbon diagram of the seven leucine-rich repeats and α-helical capping motif (gray) of InlB. (A) Sequence of the LRR domain of InlB studied here. Secondary structure assignments (x = any residue, a = α-helix, b = β-strand, h = 310 helix, s = small residue) are taken from Marino et al. (1999). The single cysteine, located in the C-terminal β-strand, was replaced with a serine. Residues not visible in the crystal structure are shown in italics. (B) Ribbon diagram of the crystal structure of InlB (Marino et al. 1999). The six trans-proline residues are shown in CPK representation. The single tryptophan, located in the third leucine-rich repeat, is shown in black. Panel B was generated using PyMOL (DeLano Scientific).