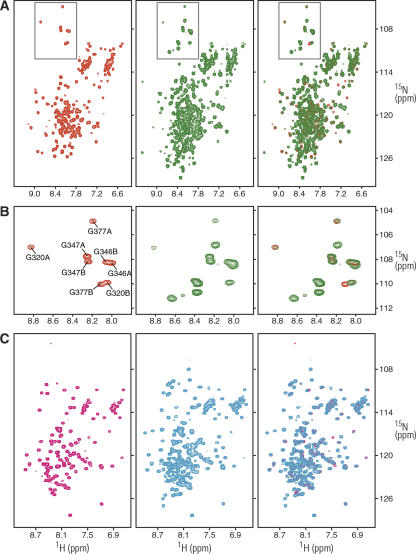
Figure 1.
NMR spectra of the N-terminal PAH domains of mSin3A and mSin3B reveal the absence of intramolecular interactions between the domains. (A) 1H-15N correlated spectra of the mSin3A PAH2 (red), mSin3A PAH1/PAH2 (green), and an overlay of the two spectra (right) recorded at 25°C in 20 mM sodium phosphate buffer (pH 6.0). Protein concentrations were 0.35 mM, and identical NMR data acquisition, processing, display, and contouring threshold parameters were used. (B) Expanded plots corresponding to the glycine region (gray boxes) of the spectra shown in panel A. The assignments for the glycine residues in the two conformers (designated A and B) are shown (Zhang et al. 2006). (C) 1H-15N correlated spectra of mSin3B PAH2 (magenta), mSin3B PAH1/PAH2 (cyan), and an overlay of the two spectra (right) recorded at 25°C in 20 mM sodium phosphate buffer (pH 6.0). Protein concentrations were 0.24 mM, and identical NMR data acquisition, processing, display, and contouring threshold parameters were used.