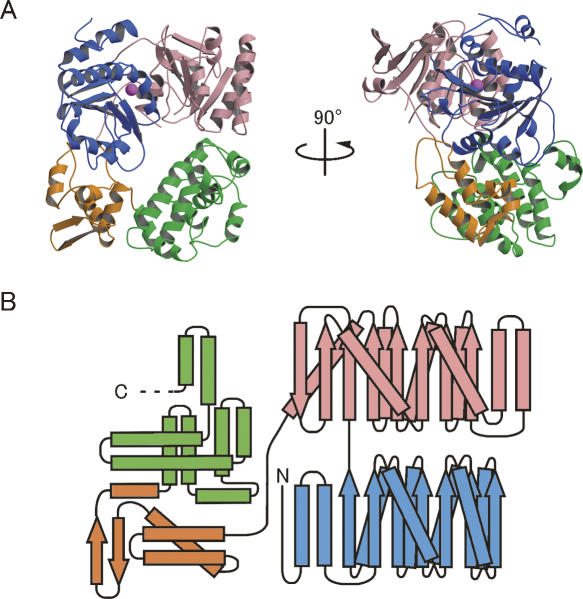
Figure 5.
Overall structure of the archaeal Ski2p-like protein Ph1280p in P. horikoshii. (A) Orthogonal views of a ribbon diagram of the crystal structure of Ph1280p. The protein comprises four subdomains; the N-terminal subdomains N1 (blue) and N2 (pink) form the helicase core domain, whereas the C-terminal subdomains C1 (orange) and C2 (green) predominantly fold into the α-helical structure, having WH-fold and HhH-fold, respectively. A possible Mg2+ ion bound to the side chain of Asp145 in motif II is shown as a magenta sphere. (B) A topological diagram showing the secondary structure of Ph1280p. α-Helices and β-strands are indicated by rectangles and arrows, respectively. Color coding is the same as in Figure 1. All figures were prepared using Molscript (Kraulis 1991) and Raster3D (Merritt and Bacon 1997).