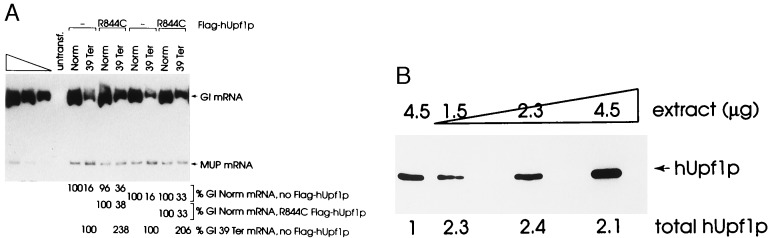
Figure 4.
Stable expression of hUPF1 R(844)C cDNA in HeLa cells abrogates the nonsense-mediated decrease in the abundance of β-globin mRNA. (A) A HeLa cell line stably transfected with pFlag-hUPF1-IRES1neo R844C was transiently transfected with 25 μg of pmCMV-Gl test plasmid (either Norm or 39Ter) and 18 μg of the phCMV-MUP reference plasmid. Total-cell RNA was purified (29, 30), and RT-PCR and PhosphorImaging were used to quantitate Gl and MUP transcripts. Gl and MUP mRNAs generate 486-bp and 199-bp products, respectively. The level of mRNA from each mCMV-Gl allele was normalized to the level of MUP mRNA. Normalized values for Gl mRNA then were calculated as a percentage of the normalized value of Gl Norm mRNA either in the presence (R844C) or absence (−) of the Flag-hUPF1 expression vector, each of which was considered as 100. Percentages differed between two independently performed experiments by no more than 5%. Normalized values for Gl 39Ter mRNA were also calculated as a percentage of the normalized value of Gl 39Ter mRNA in the absence (−) of the Flag-hUPF1 expression vector. (B) Immunoblot analysis of 4.5 μg of protein from untransfected HeLa cells and serial dilutions of 4.5 μg of protein from the HeLa cell line stably transfected with Flag-hUPF1-IRES1neo harboring the R844C mutation. Dilutions were analyzed to establish that there is a linear relationship between the amounts of input protein and immunoreactive hUpf1 protein. Total Upf1p is the sum of R844C hUpf1p and HeLa cell hUpf1p in 4.5 μg of total-cell protein, where the level of hUpf1p was considered as 1.