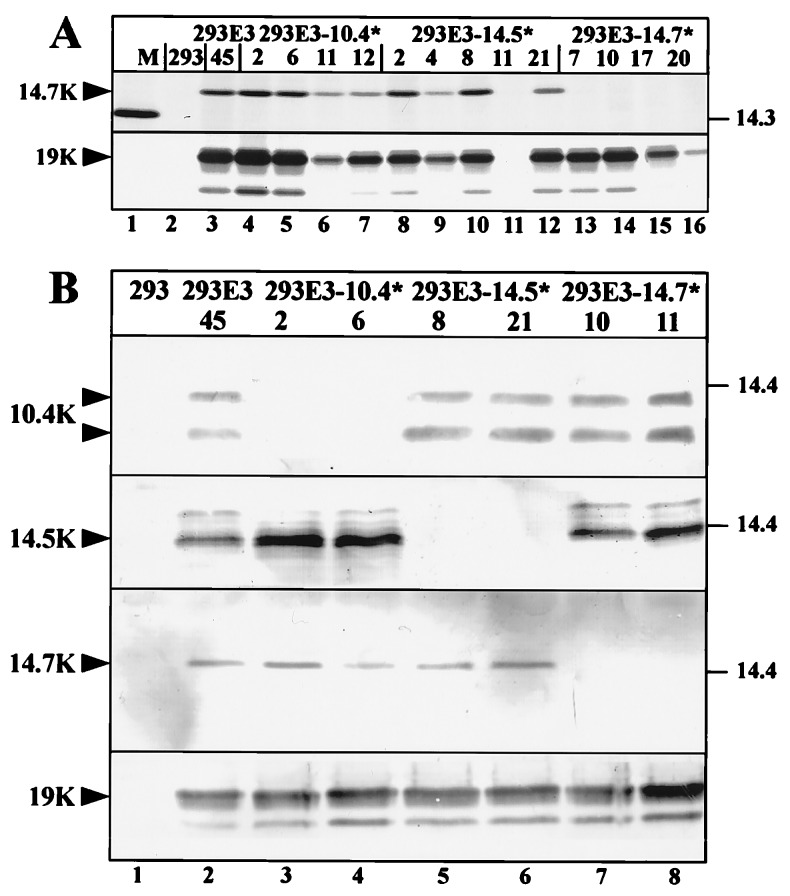
Figure 2.
Selective knock-out of E3B protein expression. (A) Immunoprecipitation of E3/14.7K and E3/19K from 293 cells (lane 2), 293E3–45 cells containing the wild-type Ad2 E3 transcription unit (lane 3), 293E3–10.4* cell clones (lanes 4–7), 293E3–14.5* cell clones (lanes 8–12) and 293E3–14.7* cell clones (lanes 13–16). 5 × 106 cells were labeled with 100 μCi/ml (1 Ci = 37 GBq) of each [35S]methionine and [35S]cysteine for 3 h, 1 ml detergent lysates were immunoprecipitated with mAb Tw1.3 against the E3/19K protein or with a rabbit antiserum against the E3/14.7K protein. Immunoprecipitates were subjected to SDS/PAGE on an 11.5% to 13.5% gradient gel. The migration positions for the 14.7K and the 19K proteins are indicated by arrowheads. Molecular masses of the marker proteins (M) are given in kDa. 293E3–14.5*-11 is a G418-resistant clone that does not express E3 proteins. (B) Western blot analysis of mutant cell lines. Immunoprecipitates of detergent extracts from similar number of cells (1.5 × 107) were separated by SDS/PAGE and analyzed by western blotting with the same antisera used for immunoprecipitation. E3/19K was immunoprecipitated with mAb Tw1.3 and probed with an E3/19K specific rabbit serum (18). Specific bands are indicated.