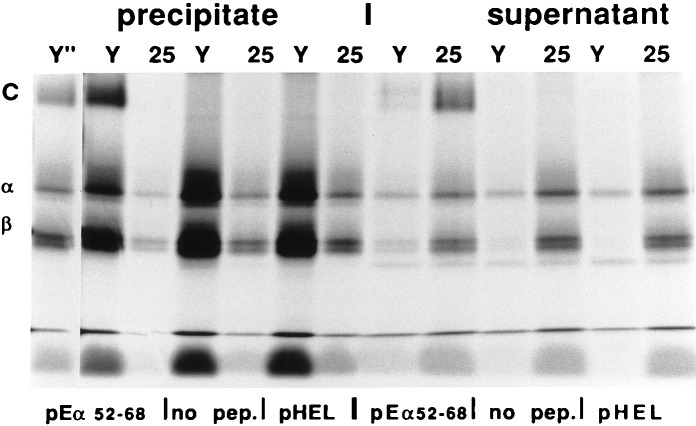
Figure 2.
Failure of mAb 25-9-17 to interact with Ab–pEα is not due to interference by poly-Gly linker: this mAb also fails to precipitate Ab molecules loaded with pEα in vitro. Ab–hCLIP molecules metabolically labeled with [35S]methionine and affinity-purified from T2 cell line were reloaded with peptides in vitro in the presence of HLA-DM and precipitated with mAb Y3JP (lanes Y) and 25-9-17 (lanes 25) (PRECIPITATE). Part of the supernatant from the precipitation reaction was also electrophoresed on the gel (SUPERNATANT), which shows material nonabsorbed by a mAb. SDS-stable C dimers were only detected in the presence of Eα 52–68 peptide but not if no peptide or HEL 46–61 (does not bind Ab) were used. Because Y3JP appears to be a better binder than 25-9-17, a shorter exposure of the Y3JP precipitation of Ab-pEα is shown (Y" lane at far left).