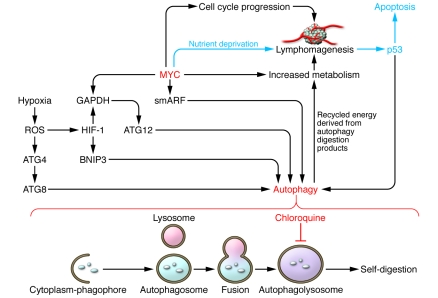
Figure 1. Inhibition of autophagy by chloroquine blocks recycling of energy and MYC-mediated lymphomagenesis.
Activated MYC induces lymphomagenesis (depicted as a cluster of cells with neovasculature) via activation of genes involved in cell cycle progression, which increases metabolic demand. This demand is in part met by the upregulation of MYC target genes involved in energy metabolism. MYC also induces p53, which triggers apoptosis in the setting of nutrient deprivation, unless the autophagy pathway can be activated to fulfill the energetic needs of the MYC-transformed cancer cell. MYC is depicted to activate a small mitochondrial isoform of ARF known as “smARF,” which translocates into mitochondria, triggering autophagy. Hypoxia is depicted to increase ROS, which in turn increase the levels of HIF-1. HIF-1 induces BNIP3 and, with MYC, can induce GAPDH. Both BNIP3 and GAPDH have been shown to regulate autophagy (14, 17). In addition, ROS modulate ATG4, permitting induction of autophagy via ATG8 (19). Autophagy occurs through assembly of cytoplasmic components within a membranous phagophore, which results in the formation of an autophagosome. A lysosome fuses with an autophagosome to form an autophagolysosome. In the report by Maclean and coworkers in this issue of the JCI, chloroquine is demonstrated to inhibit the final step in the autophagy pathway — the degradation/digestion of cargo within the autophagolysosome in order to provide recycled energy for the cell — thereby preventing MYC-mediated lymphomagenesis (4).