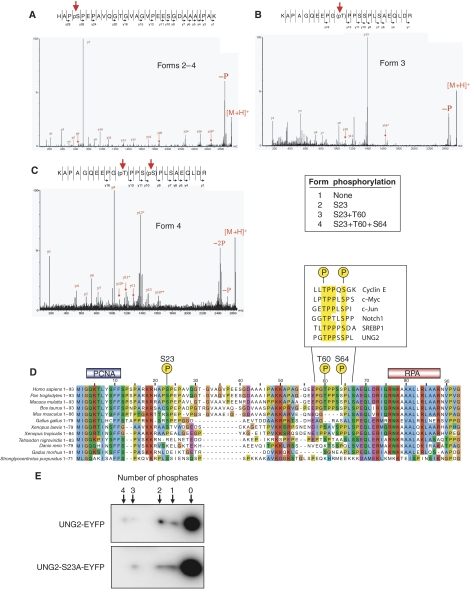
Figure 2.
Characterisation of phosphorylation sites in UNG2 by MALDI Q-TOF MS/MS. (A) Spots 2–4 contain phosphate on Ser23. (B) Spot 3 contains phosphate on Thr60. (C) Spot 4 contains phosphates at both Thr60 and Ser64. (D) Alignment of N-terminal amino-acid sequences in UNG2 from higher eukaryotes using ClustalX (Thompson et al, 1997). Individual residues are coloured according to ClustalX colour coding. Highest degree of conservation is observed in the regions corresponding to the PCNA- and RPA-binding motifs of hUNG2. Among the serines and threonines outside these motifs, S23, T60 and S64 are the most conserved. The boxed sequences in (D) illustrate phosphodegrons in other human proteins, which share sequence homology to UNG2. (E) Phosphorylation of T60/S64 may occur in the absence of S23 phosphorylation. WT UNG2 and UNG2 S23A were expressed as EYFP fusion proteins in HeLa cells, and different phosphoforms visualised subsequent to 2D PAGE and western analysis using antibodies against EYFP.