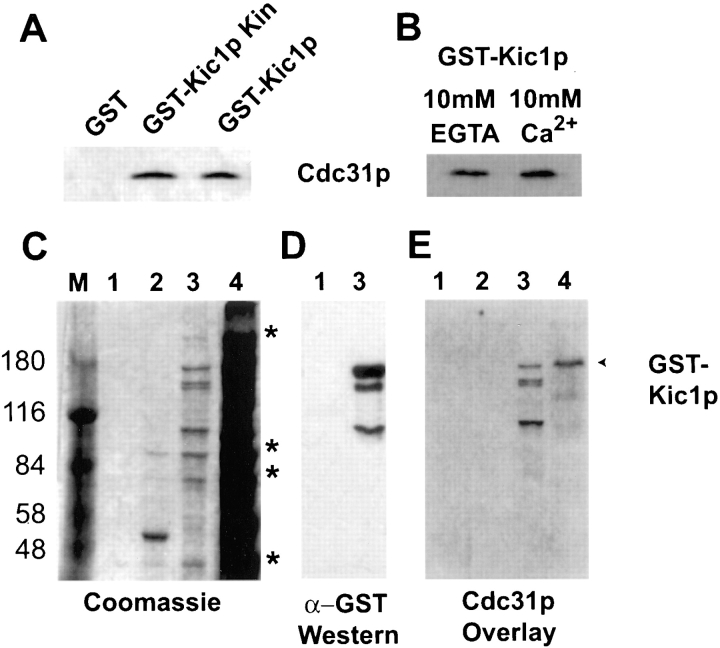
Figure 2.
Cdc31p binds Kic1p directly and is part of a larger complex. (A) Cdc31p coprecipitates with Kic1p. GST, GST catalytic domain, and GST full-length Kic1p fusion proteins were purified from yeast (strains MY4124, MY4125, and MY4126, respectively) using glutathione–agarose under native conditions, separated on denaturing gels, and then Western blotted with anti-Cdc31p antibodies. Cdc31p band is indicated for part A and B. (B) Cdc31p interaction with Kic1p is calcium independent. The full-length Kic1p fusion protein was purified from MY4126 in the presence of 10 mM EGTA or 10 mM calcium. Equal amounts of purified Kic1p were run on a gel and Western blotted with anti-Cdc31p antibodies. (C) Coomassie staining of the pure GST–Kic1p fusion proteins as well as copurifying proteins. Purified GST (lane 1, strain MY4124), GST–catalytic domain (lane 2, strain MY4125), and GST full-length fusion proteins (lane 3, MY4126) were separated on an 8% denaturing gel. Lane 4 contains the total yeast extract from MY4126 expressing GST fused to full-length Kic1p. Copurifying peptides of 200, 80, 70, and 40 kD are indicated with asterisks. The GST protein is not observed because it is <30 kD and therefore ran off the gel. Higher percentage gels were run to monitor GST purification (not shown). M, protein molecular weight markers. (D) Anti-GST Western blotting showing full-length GST–Kic1p and several degradation products. Lanes marked 1 and 3 are as in C. (E) Cdc31p binds to full-length GST– Kic1p and its degradation products. Fusion proteins were transferred to membrane and a gel overlay was performed with radiolabeled Cdc31p (Biggins and Rose, 1994). Lanes are marked as in C.