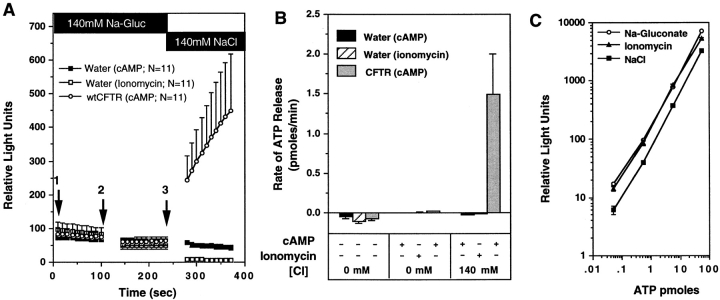
Figure 2.
Absence of ATP efflux after activation of Ca+-activated Cl− conductance in oocytes. To assess whether membrane potential changes due to opening of Cl− channels in oocytes could nonspecifically facilitate ATP efflux, the rates of ATP efflux in cAMP/forskolin stimulated CFTR cRNA- injected oocytes, and ionomycin-treated water-injected oocytes were compared. A demonstrates the mean (± SEM) relative light units produced from oocytes derived from two frogs after exposure to the following sequence of buffers: (1) 140 mM Na-gluconate/HPBR; (2) 140 mM Na-gluconate/HPBR + (200 μM 8-cpt-cAMP, 2.5 μM forskolin, or 10 μM ionomycin); and (3) 140 mM NaCl/HPBR + (200 μM 8-cpt-cAMP, 2.5 μM forskolin, or 10 μM ionomycin). Buffer changes are denoted by arrows, and the treatment conditions are indicated in the legend. B depicts the mean (± SEM) rate of ATP efflux for each buffer condition as calculated from the slope of relative light units vs. time (for the 100-s reading) for each individual oocyte. ATP concentration curve standards were used to calibrate rates in terms of pmoles/min (1 pmole ATP = 90 light units). All oocytes analyzed were included in these results. To demonstrate that ionomycin has no effect on the activity of luciferin–luciferase, we compared standard curves for ATP in the presence of buffers and agonists used for the above study (C). Ionomycin standard curves were measured in Na-gluconate buffers.