Figure 4.
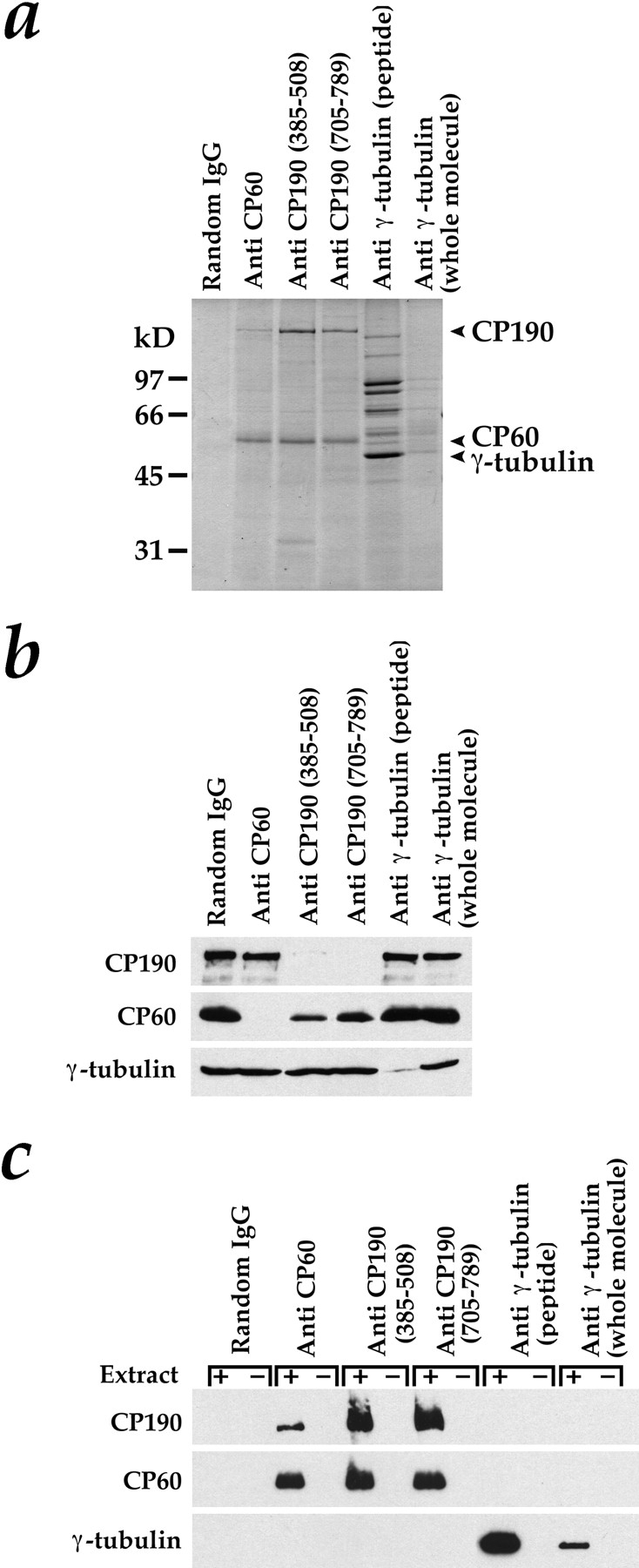
Tests for complex formation by immunoprecipitation. Since Drosophila γ-tubulin is the same size as IgG heavy chain, the immunoprecipitations in a–c were carried out with special care to avoid any IgG contamination in the pellets (see Materials and Methods). (a) Immunoprecipitation pellets after separation by SDS-PAGE on an 11% gel and staining with Coomassie blue. Anti-CP60 immunoprecipitated CP60 and a small fraction of the CP190. Both anti-CP190 antibodies immunoprecipitated CP190 and a large percentage of the CP60. Antibodies to γ-tubulin immunoprecipitated γ-tubulin and a group of γ-tubulin–associated proteins that are components of the Drosophila γTuRC. The anti-γ–tubulin COOH-terminal peptide antibody was much more effective in immunoprecipitations than the antibody made to the whole γ-tubulin molecule. (b and c) Western blots to detect CP190, CP60, and γ-tubulin in immunoprecipitation supernatants (b) and pellets (c). In c, beads were incubated in the presence (+) or absence (−) of extract to control for antibody contamination in the pellets.
