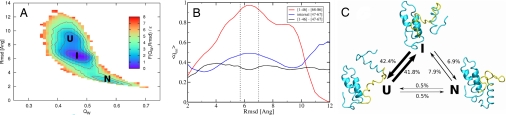
Fig. 1.
Characterization of the IM7 wild type folding thermodynamics and its intermediate features. (A) Free energy (in units of ε) of the IM7 at T̄ = 1.0 as a function of QW and rmsd. The wells correspond to the unfolded (U), intermediate (I), and native (N) states. (B) The average fraction of nonnative contacts as a function of the rmsd. In the red curve, we consider only the interactions between the first half of the protein (residues 1–46) with the fourth helix (residues 68–86). The blue curve shows the same ratio but only for the contacts internal to the helix III region (residues 47–67), and the black curve represents the contacts between the first half and the helix III region. Also displayed with vertical dotted lines are the boundaries used to define the intermediate state. (C) The percentage of the transitions observed between the three states U, I, and N suggests the intermediate to be on-pathway in the folding reaction. The states are operatively defined in terms of QW and rmsd as U = [0.43; 0.47] × [8.4; 9.7], I = [0.45; 0.51] × [5.7; 7.0] and U = [0.55; 0.60] × [2.5; 3.3], and a transition is counted every time the trajectory in QW, rmsd space leaves one state and enters another without passing through the third. The percentage of jumps are calculated out of 1,890 observed transitions during 40 independents simulations at T̄ = 1.0. Three representative snapshots of each state also are shown, with the helix III region highlighted in yellow.