According to Pelis (1), “Blood is one of man's most compelling and omnipresent symbols. It signifies the opposition between life and death, health and sickness, power and powerlessness.” Blood and RBC transfusion, even done autologously, is a well proven treatment that, like the administration of aspirin, had never undergone the kind of rigorous, controlled clinical trials now required by the U.S. Food and Drug Administration.
In a recent issue of PNAS, two papers (2, 3) provide new insights into the detrimental effects of storage on human RBCs.
Some of the properties of stored blood have long been known to be problematic in the realm of blood transfusions. For example, we know that the oxygen affinity of stored blood rises during the storage period (4) and that intracellular allosteric regulators, notably 2,3-bisphosphoglyceric acid (DPG) and ATP, are depleted during storage. During the Vietnam era, U.S. Navy physician C. Robert Valeri and N. M. Hirsch (5) showed that “spiking”-stored RBCs with DPG and ATP precursors led to significant improvements in cardiovascular function.
These findings prompted a significant effort in the following decade that was aimed at changing the oxygen affinity of RBCs with the goal of increasing the efficacy conveyed by transfusion of stored blood. From 1975 to 1980, I worked as an American Heart Established Investigator with the goal of tailor-making transfusion blood with specific oxygen-binding properties. Other scientists went as far as to fuse RBCs with phospholipid vesicles, or reversibly hemolyze RBCs, filling them with Hb and inositol hexaphosphate, a powerful nonmammalian heterotropic effector (6).
In 1987, our understanding of the pathways of vascular regulation in human physiology took a surprising turn. Independent studies completed by Ignarro et al. (7) and Furchgott and Zawadzki (8) suggested endothelium-derived relaxation factor and nitric oxide to be one and the same. Ignarro et al. (9) took this hypothesis a step further by building on the stereochemical model of Hb, which was originally proposed by Perutz (10). The conformational transition from unliganded to liganded Hb is accompanied by the iron atoms moving from out to within the plane of the heme, triggering the transition from T- to R-state Hb. Ignarro et al. suggested a similar structural transition to be induced by NO on binding to heme iron within soluble guanylate cyclase, leading to its activation and ultimately to smooth muscle relaxation.
Subsequent work suggested that the model of Ignarro et al. was correct. However, for NO-mediated activation of guanylate cyclase to occur, it would need to reach the vessel wall. At the time, NO transit from RBCs to the vessel wall seemed paradoxical, given what was then known about NO binding to Hb. NO binds to Hb ferrous hemes ≈1 million times more tightly than oxygen, yet the presence of ≈750 g of intracellular Hb did not seem to interfere with NO-dependent vasoactivity. Extracellular Hb, however, was found to interfere with NO vasoactivity, inducing marked hypertension subsequent to its infusion (11). The profound clinical efficacy of transfusions cannot be understated. However, in some scenarios, blood transfusions have been found to be harmful to patients (12). In these cases, the underlying etiology determining negative outcome is poorly characterized and does not seem to be caused exclusively by any of the time-dependent changes that are known to occur in stored blood.
The function of Hb in transporting oxygen and carbon dioxide is well appreciated within the scientific community. Yet, in 1952, Linus Pauling (13) made a statement that diverged from the conventional wisdom of Hb function: “It may well be that the hemoglobin molecule carries out other functions, but not so much is known about them as about these functions of assisting in the transport of oxygen from the lungs to the tissues and of carbon dioxide from the tissues to the lungs.” How right he was.
In hindsight, this statement seems to foreshadow the revolutionary discoveries published in a 1996 article (14), describing reversible binding of NO to β93C, Cys-93 of Hb β chains (14). This was proposed to be a major functional interaction between NO and Hb. Additionally, a cycle of NO binding that captured and released NO from RBCs through reversible formation of Hb-β93C-S-nitrosothiol (SNO-Hb) was suggested, possibly explaining the NO paradox discussed above. Since that time, scientists have shown that this cycle is a fundamental part of respiratory gas transport in the tissues, whereby hypoxic tissues signal SNO-laden RBCs to unload vasoactive, allosterically bound β93C-SNO and oxygen more or less simultaneously (14–16).
In a recent issue of PNAS, two groups at the Duke University Medical Center (2, 3) have built on this work by analyzing the time-dependent changes that occur in stored blood. During these studies, each group operated independently of the other, yet still converged on similar discoveries. The first and larger group, headed by McMahon (2), tested this hypothesis: multiple components work together in leading to the “storage lesion” in RBC physiological functions critical to oxygen delivery, particularly deformability and RBC-dependent vasoactivity. In the second group, Stamler and colleagues (3) tested the following hypothesis: impaired vasoregulation associated with administration of banked blood results partly from losses in NO bioactivity derived from RBC, but this activity can be restored. Both studies beautifully accomplish the aims and confirm the hypotheses. What follows here blends the results from both articles, the first that uses RBCs and the second that uses banked blood, with the hope that an integrated picture of the results is obtained.
The methods used by both groups were identical, such that a valid comparison of the results from each study can be made. Total Hb-bound NO rapidly decreased over 3 h and remained throughout the duration of each study. The Stamler group (3) describes methodology for chemical restoration of SNO levels in stored RBCs, a process that not only returns SNO to these cells but also allows their ability to participate in functional hypoxic vasodilation. In both articles (2, 3), the authors point out that renitrosylation can enhance cardiac oxygen delivery via increased blood flow and, accordingly, protect against vasoconstrictor and thrombogenic stimuli.
An important message to note is that the relative numbers of Hb molecules in the RBCs that carry out the vasoactive function are very small, comprising ≈0.1% of the total. Hence, the situation is one where oxygen, as an allosteric regulator of NO donation by Hb, acts on a small subpopulation of Hb molecules to obtain the needed function of offloading vasoactive NO. This interesting situation gives the RBC an added degree of self-control in delivery of oxygen to particular sites in the body and provides another beautiful example of biochemical division of labor within single cells.
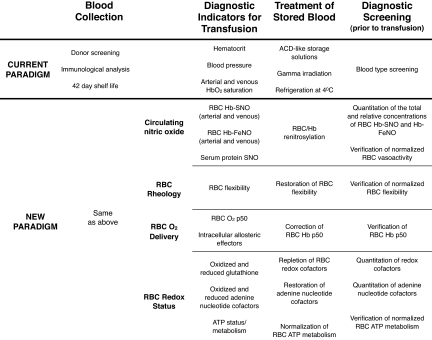
The papers (2, 3) make a clear case for pursuing clinical trials that could lead to beneficial changes in the way blood is collected, stored, and administered. Fig. 1 presents an overview of current protocols and a potential new paradigm for blood collection, diagnostic indicators for transfusion, treatment of stored blood, and testing before transfusion. Most of what is proposed in the new paradigm is already well within the capabilities of standard clinical laboratories. The development and deployment of a clinical apparatus that could perform all of these measurements could likely be achieved at a minimal expense, such that its use would not be limited to tertiary care centers. The cost of these diagnostics could be economically feasible for small rural hospitals and even less-developed countries, providing all of the information needed to evaluate stored blood immediately before transfusion.
Fig. 1.
An overview of current protocols and a potential new paradigm for blood collection, diagnostic indicators for transfusion, treatment of stored blood, and testing before transfusion.
Hb is, by far, the most abundant component of the RBC. However, it does not function without supporting cellular components, proteins, enzymes, and cofactors. The discoveries described in refs. 2 and 3 document biochemical and functional changes that accompany RBC storage, even under conditions that represent the current American standards of practice. It will be fascinating to see whether the Hb changes documented here are unitary or whether they are a part of a physiological molecular concert, wherein Hb plays a role. The extent to which this is true and whether Hb is the conductor, first violin, or part of the timpani remains to be seen. It might even turn out that the audience, i.e., the tissues of the organism, is just as important as the orchestra. I, for one, eagerly look forward to hearing and reading the rest of the music.
Footnotes
References
- 1.Pelis K. In: Blood: Art, Power, Politics, and Philosophy. Bradburne JM, editor. New York: McGraw–Hill; 2001. p. 771. [Google Scholar]
- 2.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Proc Nat Acad Sci USA. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. Proc Natl Acad Sci USA. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valtis DJ. Lancet. 1954;266:199–224. doi: 10.1016/s0140-6736(54)90978-2. [DOI] [PubMed] [Google Scholar]
- 5.Valeri CR, Hirsch NM. J Lab Clin Med. 1969;73:722–733. [PubMed] [Google Scholar]
- 6.Gersonde K, Nicolau C. Naturwissenschaften. 1979;66:567–570. doi: 10.1007/BF00368811. [DOI] [PubMed] [Google Scholar]
- 7.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 8.Furchgott RF, Zawadzki JV. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro LJ, Adams JB, Horwitz PM, Wood KS. J Biol Chem. 1986;261:4997–5002. [PubMed] [Google Scholar]
- 10.Perutz MF. Nature. 1972;237:495–499. doi: 10.1038/237495a0. [DOI] [PubMed] [Google Scholar]
- 11.Hess JR, MacDonald VW, Brinkley WW. J Appl Physiol. 1993;74:1769–1778. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 12.Tinmouth A, Fergusson DA, Yes IC. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 13.Pauling L. Proc Am Philos Soc. 1952;96:556–565. [Google Scholar]
- 14.Jia L, Bonaventura C, Bonaventura J, Stamler J. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 15.Gow AJ, Luchsinger BP, Pawloski JR, Singel DJ, Stamler JS. Proc Natl Acad Sci USA. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, et al. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]