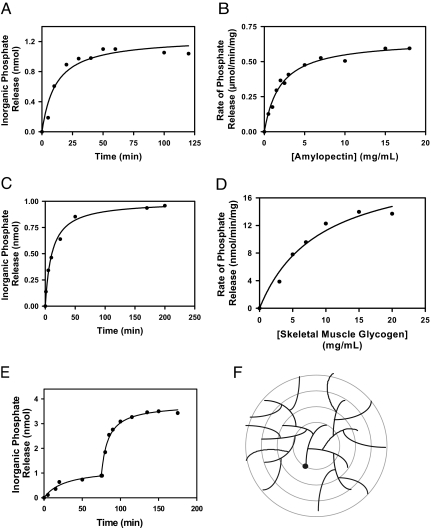
Fig. 1.
Dephosphorylation of amylopectin and glycogen by laforin. The data shown are representative of at least five independent experiments. (A) Time-dependent release of phosphate from potato amylopectin (0.5 mg/ml) by purified, recombinant laforin (2.5 μg/ml). At the indicated times, the reaction was terminated by the addition of N-ethylmaleimide, and the inorganic phosphate was determined. (B) Concentration dependence of the rate of dephosphorylation of amylopectin by laforin. The reaction was for 15 min in the presence of 2.5 μg/ml laforin, at which time the reaction was close to linear. The apparent Km for amylopectin was 1.5 mg/ml. (C) Time-dependent release of phosphate from rabbit skeletal muscle glycogen (5 mg/ml). Laforin was present at 25 μg/ml. (D) Concentration dependence of glycogen dephosphorylation by laforin. Laforin was present at 25 μg/ml, and the reaction time was 40 min. The apparent Km for glycogen was 4.5 mg/ml. (E) Dephosphorylation of rabbit skeletal muscle glycogen in the presence of α-amylase and amyloglucosidase. Glycogen (5 mg/ml) dephosphorylation by laforin (25 μg/ml) was allowed to proceed until the reaction was essentially complete, at which time α-amylase (0.3 mg/ml) and amyloglucosidase (0.3 mg/ml) were added. Subsequent phosphate release was monitored. (F) Model for glycogen structure. Glycogen is believed to exist as a series of concentric shells of glucose residues, so that inner tiers would not be on the surface of the molecule. A full-size molecule would consist of 12 tiers.