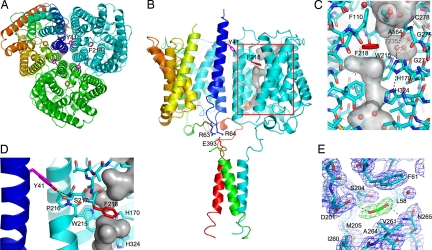
Fig. 2.
Functionally relevant sites within the N. europaea Rh protein. (A) Periplasmic view of the N. europaea Rh protein along the three-fold axis. Two key residues implicated in channel opening are colored in violet (Tyr-41) and red (Phe-218). Subunits are colored in cyan, green, and rainbow [from blue (N terminus) to red (C terminus)]. (B) Side view of the N. europaea Rh protein emphasizing the key residues implicated in partner protein-mediated channel gating. In the cyan subunit the surface of the channel is shown together with the location of the putative CO2 binding site. Tyr-41 on helix M1 from one subunit (in rainbow) is proposed to regulate the extent of kinking of transmembrane helix M6 on the adjacent subunit (in cyan). The position of Tyr-41 in turn is linked to the position of helix M1. Partner protein binding to the C-terminal α-helix is proposed to alter the position of the M1 helix via salt bridge interactions between Glu-393 on the C-terminal helix and Arg-63 and Arg-64 on helix M1. (C) Key channel residues on the periplasmic side of the channel. The protein is depicted in stick form with carbon atoms colored in cyan and the remaining atoms in CPK. Waters and the putative ethylene glycol are shown in ball-and-stick form. Potential cavities are highlighted with a transparent surface colored in gray. For the unique hydrophilic pocket adjacent to Phe-218, the hydrogen-bonding interactions of its three waters are depicted as black dotted lines. (D) Interactions between Tyr-41 and the M6 helix of the adjacent subunit. Potential hydrogen bonds between Tyr-41 and Ser-217 are shown as black dotted lines. (E) Weighted density maps surrounding the CO2 binding site, 2mFO–DFC (in black, contoured at 1.0σ) and mFO–DFC (in green, contoured at 3.0σ, and in red, contoured at −3.0σ) with terms as determined by REFMAC5 (36).