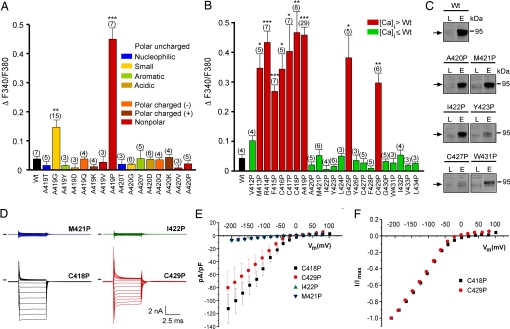
Fig. 3.
Effects of amino acid substitutions in TM5 of TRPML3. (A) Effect of the substitution of alanine at positions 419 and 420 in murine TRPML3 by various amino acids on [Ca2+]i. Note that only proline and glycine substitution at position 419 significantly led to an increase of [Ca2+]i. Shown are mean values ± SEM with n in parentheses. All experiments were performed 10–15 h after transfection. (B) Effect on [Ca2+]i of proline substitution of the residues predicted to constitute TM5 of murine TRPML3. Shown are mean values ± SEM with n in parentheses. Nine (red) of the 23 mutant isoforms showed significantly elevated basal intracellular calcium levels compared with wild-type TRPML3 (Wt). V412P is not part of the predicted TM5. ***, P < 0.0001; **, P < 0.001; *, P < 0.01 (Student's t test, unpaired, comparison with wild type). (C) Surface biotinylation analysis. TRPML3 wild-type protein (Wt) and the mutant isoforms are present in the plasma membrane. L indicates input load (1% of total), and E indicates elution from neutravidin beads of surface-biotinylated TRPML3 visualized by Western blot. (D) Voltage-clamp recording from mutants as listed, expressed in HEK293 cells. Voltage protocol consists of 20-mV incremental steps between −200 and +80 mV. Inward rectifier current is present in mutant TRPML3-expressing cells that showed elevated calcium and is not present in those where calcium was not elevated. Black bars indicate zero current line. (E) Steady-state current–voltage plots generated from data in D. (F) Current–voltage plots normalized to current at −200 mV to demonstrate similar relationships between mutants.