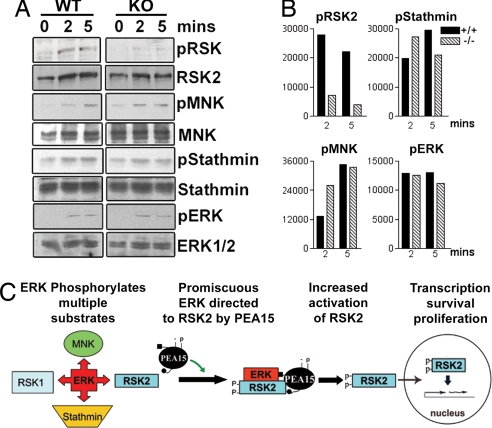
Fig. 5.
PEA-15 does not enhance ERK phosphorylation of all substrates. Equal numbers of lymphocytes from WT and PEA-15-null mice were stimulated with PMA for the indicated times. (A) Lysates were immunoblotted to detect phosphorylated RSK2 (S386), MNK, Stathmin, and ERK. Total RSK2, MNK1, Stathmin, and ERK1/2 levels were detected as controls for protein loading. (B) Densities of bands for phosphorylated RSK2, MNK, Stathmin, and ERK1/2 were quantitated, and the values are shown relative to controls without PMA. (C) PEA-15 is a regulated scaffold for ERK activation of RSK2. ERK recognizes many substrates. In cells expressing PEA-15, a fraction of cellular ERK is targeted specifically to RSK2. PEA-15 enhances the association of ERK with RSK2, resulting in increased phosphorylation and activation of RSK2. The scaffold complex is regulated by kinases such as PKC through phosphorylation of PEA-15.