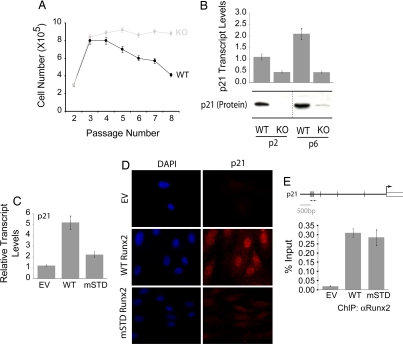
Fig. 2.
Runx2-deficient osteoblasts exhibit growth advantage ex vivo owing to the loss of p21WAF1/CIP1. (A) Primary diploid WT and KO osteoblasts were cultured ex vivo according to the standard 3T3 protocol. Cells were counted at each passage and replated at the initial plating density (i.e., 3 × 105). The line graph represents the number of cells at the time of each passage. WT osteoblasts showed a considerable decrease in their rate of proliferation as a function of increasing passage. (B) Total cellular RNA was isolated from WT and KO osteoblasts grown at the indicated passages and was subjected to quantitative RT-PCR to assess the expression of p21WAF1/CIP1, a CDK2 inhibitor. KO osteoblasts showed a significant decrease in the expression of the p21WAF1/CIP1 transcript. KO osteoblasts grown on passage 3 were infected with retroviruses carrying the empty vector (EV) or cDNAs for the WT or an mSTD of Runx2. Cells were harvested 48 h after infection for total cellular RNA isolation, ChIP, or immunostaining for p21WAF1/CIP1. (C) As shown, the WT Runx2 restored the expression of p21WAF1/CIP1 in KO osteoblasts, whereas the mSTD Runx2 failed to enhance the p21WAF1/CIP1 expression. (D) Immunostaining revealed that the p21WAF1/CIP1 protein levels also were restored by the WT protein but not the mSTD Runx2 protein. (E) Both the WT and mSTD Runx2 proteins occupied the p21WAF1/CIP1 promoter in vivo as assessed by ChIP assay.