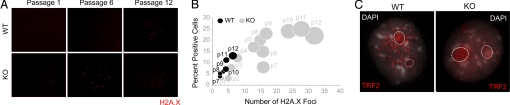
Fig. 3.
Runx2-deficient osteoblasts exhibit hallmarks of genomic instability. (A) WT and KO osteoblasts grown at the indicated passages were subjected to immunofluorescence. Spontaneous DNA damage was assessed by staining cells with an antibody against the phosphorylated form of histone variant H2A.X (γH2A.X). (B) The increased incidence of spontaneous DNA damage in KO osteoblasts is quantitatively represented in a bubble graph. One hundred cells were counted for each of WT and KO osteoblasts. Cells that showed γH2A.X staining were scored as positive and are represented in the graph as percentage of positive cells (the y axis). In each positive cell, the number of γH2A.X foci also was counted and is represented on the x axis. Thus, the size of each bubble reflects the number of positive cells at each passage, as well as the number of γH2A.X foci in each positive cell. (C) WT and KO osteoblasts grown on gelatin-coated coverslips at passage 5 were immunostained for TRF2, a regulatory protein required for the maintenance and integrity of telomeric ends. WT osteoblasts exhibited the expected punctate staining of TRF2, whereas KO cells showed diffused TRF2 localization, indicating a loss of telomeric integrity. TRF2 is a known regulator of ribosomal gene expression and also is localized to nucleoli (demarcated here by dotted line circles).