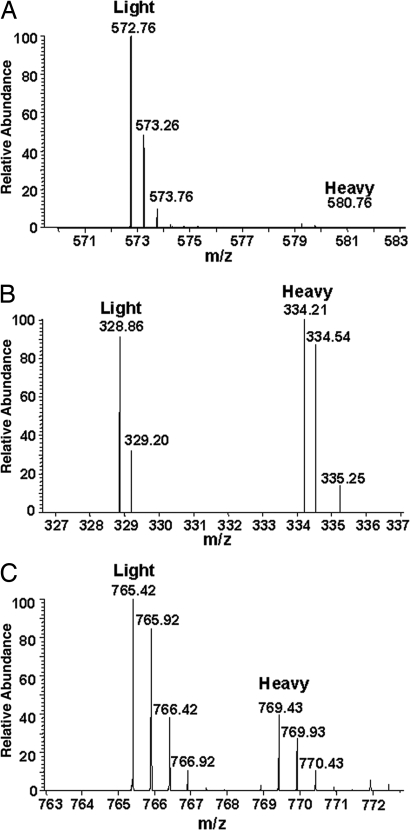
Fig. 2.
Relative SILAC quantification of phosphorylated peptides between ER and cell-surface forms of Kv1.2. (A) A doubly charged peptide representing doubly phosphorylated Kv1.2 at pS440/pS441 (IPpSpSPDLKK) was abundant in the sample from the cell-surface Kv1.2 pool (Light, peak at m/z 572.76) but was not detected in the samples from the ER Kv1.2 pool (Heavy, lack of peak at m/z 580.76). (B) The corresponding triply charged unphosphorylated peptide (IPSSPDLKK) was found with similar mass peak intensities in both the cell surface (Light, peak at m/z 328.86) and ER (Heavy, peak at 334.21) Kv1.2 pools. (C) A representative doubly charged nonphosphopeptide (TLAQFPETLLGDPK) from Kv1.2 demonstrates the overall ratio (≈2:1) of Kv1.2 between cell surface (Light, peak at m/z 765.42) and ER (Heavy, peak at m/z 769.43) samples applied for SILAC analysis.