Abstract
In Neurospora, metabolic oscillators coexist with the circadian transcriptional/translational feedback loop governed by the FRQ (Frequency) and WC (White Collar) proteins. One of these, a choline deficiency oscillator (CDO) observed in chol-1 mutants grown under choline starvation, drives an uncompensated long-period developmental cycle (≈60–120 h). To assess possible contributions of this metabolic oscillator to the circadian system, molecular and physiological rhythms were followed in liquid culture under choline starvation, but these only confirmed that an oscillator with a normal circadian period length can run under choline starvation. This finding suggested that long-period developmental cycles elicited by nutritional stress could be masking output from the circadian system, although a caveat was that the CDO sometimes requires several days to become consolidated. To circumvent this and observe both oscillators simultaneously, we used an assay using a codon-optimized luciferase to follow the circadian oscillator. Under conditions where the long-period, uncompensated, CDO-driven developmental rhythm was expressed for weeks in growth tubes, the luciferase rhythm in the same cultures continued in a typical compensated manner with a circadian period length dependent on the allelic state of frq. Periodograms revealed no influence of the CDO on the circadian oscillator. Instead, the CDO appears as a cryptic metabolic oscillator that can, under appropriate conditions, assume control of growth and development, thereby masking output from the circadian system. frq-driven luciferase as a reporter of the circadian oscillator may in this way provide a means for assessing prospective role(s) of metabolic and/or ancillary oscillators within cellular circadian systems.
Keywords: Frequency-less oscillator, clock
Most organisms on Earth, from cyanobacteria to humans, use an endogenous circadian clock to adapt to daily oscillations of environmental cues (e.g., light, temperature). A functional clock provides adaptive advantages during evolution (1), and many metabolic and developmental processes appropriate for a certain time of day are under control of the endogenous clock. The circadian biological clock displays close to the same period length across a physiological temperature range (2). Temperature compensation in particular sets the circadian biological clock apart from other more typical chemical and biological reactions that run faster when temperature goes up.
The filamentous fungus Neurospora crassa has become a salient model system in circadian biology research since a mutant strain (bd−) was identified in the 1960s (3). The distinct spore bands formed in a rhythmic pattern in strains containing bd during growth on an agar surface have made Neurospora a tractable system in which to genetically screen for clock components. Historically, two rationales have been used: One was to identify new mutants that changed only clock properties and no other processes. The other route, based on the idea that the clock might arise from metabolic processes, was to screen already known metabolic mutants for clock phenotypes (4, 5).
Genetic screens with the first rationale identified the gene frequency (frq) (6), whose subsequent cloning opened up molecular analyses of a circadian feedback loop (7, 8). The FRQ-WCC (Frequency–White Collar Complex) oscillator (FWO) forms a central aspect of the Neurospora circadian system (9–12). In this oscillator, White Collar-1 (WC-1) and White Collar-2 (WC-2), two GATA-family transcriptional factors, drive frq expression beginning in the late subjective night (13). frq RNA levels peak in the subjective morning when FRQ protein, in complex with the FRQ-interacting RNA helicase (FRH), enters into the nucleus and acts as a negative element to inhibit WCC activity (7, 14–17). This causes frq levels to decline to a trough in the subjective evening. Mature and phosphorylated FRQ is then ubiquitinated and degraded by the proteasome pathway (18–20).
Strains lacking frq, wc-1, or wc-2 have defects in the clock and lose circadian regulation of conidiation under most conditions (21–23). Intriguingly, however, rhythms of conidiation, metabolic regulation, and gene expression can still be observed under certain conditions in the absence of the FWO (reviewed in refs. 11, 24, and 25). Those rhythms, regulated by cryptic FRQ-less oscillators (FLOs) (26), provide a new layer of complexity to clock research and are frequently mentioned as having potential roles within the circadian system (e.g., refs. 24 and 27). Although these FLOs when analyzed on their own have generally lost some or all circadian characteristics, the possibility remains that they may interact with the known intracellular cellular circadian feedback loop, contribute to robustness, or provide driven oscillations with important roles in output. The relative position(s) and importance of these metabolic oscillators within the clockwork have emerged with recent years as a salient question in the study of cellular circadian systems, of which Neurospora is a prime example.
Among the fruits of the second genetic approach to identify clock mutants, two lipid biosynthesis mutants were found to affect sporulation rhythmicity. One is cel, a mutant in fatty acid synthesis displaying an ≈40-h period rhythm of conidiation on solid medium supplemented with unsaturated fatty acids (28). The other is chol-1, whose product catalyzes the first methylation step in the conversion of phosphotidylethanolamine to phosphatidylcholine (29). The mutant shows a conidiation rhythm, controlled by a choline deficiency oscillator (CDO), with a period >50 h under choline starvation (30) that can be entrained by light to periods near its intrinsic length but not to periods within the circadian range. The rhythm is pH-compensated but not temperature-compensated (30–32). The period length of the rhythm reflects the degree of choline starvation, but the allelic states of the frq or wc genes have little or no effect on the rhythm.
We sought to probe the relationship between the CDO and FWO in the circadian system. As noted (33), there are many possibilities including but not limited to the following ones. (i) The CDO reflects a cryptic core oscillator whose output is modified by the FWO to make it circadian. (ii) The CDO interacts with the FWO to form the circadian system. (iii) The CDO is not involved in the circadian system but instead represents a metabolic oscillator that can displace the circadian system in controlling growth and development. To evaluate these possibilities, we followed the FWO and the oscillator regulating conidiation both in liquid and on solid medium under conditions of choline starvation. The results showed that the FWO still runs normally in the absence of choline supplementation with a frq-dependent and temperature-compensated rhythm with period length of ≈22 h. The data strongly suggest that the CDO affects only the conidiation rhythm and not the central circadian oscillator. It is consistent with the possibility, previously noted (33), that separate oscillators can control conidiation under different conditions. The data suggest that the CDO is a metabolic oscillator expressed or induced by choline starvation whose appearance masks output from the circadian system. It provides an alternative means, bypassing the circadian clock, to regulate development in a nutritionally deficient environment.
Results
FRQ Oscillation in Choline Depletion Condition.
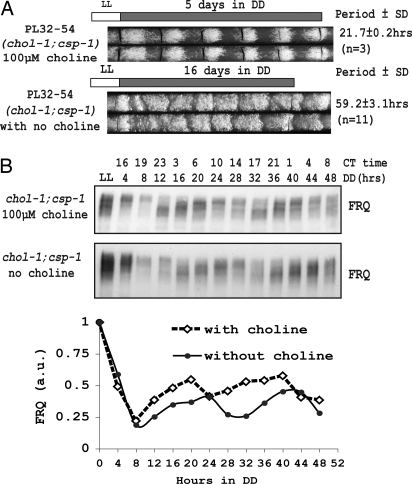
The hypothesis that the elongated chol-1 conidiation rhythm might reflect a “cryptic” circadian oscillator that was revealed only under choline deficiency (24, 31, 33) suggested that the CDO might control or influence the FWO during choline starvation. We confirmed that strain PL32-54 (bd;chol-1;csp-1) displays a characteristic elongated rhythm (period length ≈ 60 h) on race tubes without choline supplementation and a normal conidiation rhythm (period length ≈ 22 h) on race tubes supplemented with 100 μM choline (Fig. 1A), consistent with previous results (30, 32). To measure molecular rhythms in liquid cultures, bd;chol-1;csp-1 was cultured with or without choline supplementation for 12–56 h in light followed, respectively, by 48 to 4 h in darkness, so by harvest all cultures had been growing with or without choline supplementation for at least 60 h (34). Western blots revealed a normal oscillation in the level of FRQ and extent of FRQ phosphorylations in both supplemented and starved cultures (Fig. 1B).
Fig. 1.
FRQ oscillation with a circadian period (≈22 h) under conditions of choline deficiency. (A) Race tube assay of a chol-1;csp-1 strain under choline-supplemented (100 μM) and choline-starved conditions. The chol-1;csp-1 strain showed a normal circadian period length of ≈22 h with choline supplementation but an elongated period length (≈60 h) under choline starvation. The mycelial growth front was marked by a black line every day on race tubes. (B) Western blot analysis of FRQ expression in a 2-day time course from liquid culture. Oscillations of FRQ were observed in chol-1 strains in both choline-replete and choline-starved conditions.
Normal Circadian Rhythms in Liquid Cultures Lacking Choline.
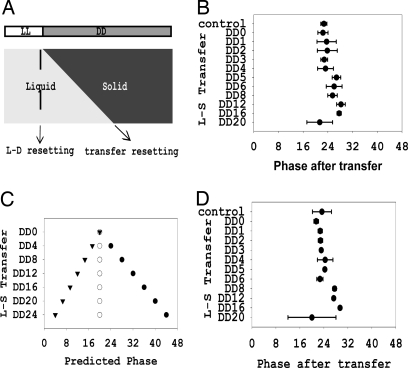
The results suggested the possibility that the FWO and CDO could both be running independently under conditions of choline deficiency. The FWO could be followed biochemically, but the CDO can be monitored only through its output in controlling growth and development. Therefore, we used an alternative rhythm assay (35) in which the phase and period length of a clock in liquid culture can be inferred from the phase of the conidiation rhythm revealed when samples are removed from liquid and allowed to express the conidiation rhythm on solid medium. We cultured chol-1 conidia without choline in liquid for different durations and placed the growing cultures on choline-supplemented race tubes. If the clock runs with a different period in liquid than on solid medium, we would expect to see the phases diverge as samples are cultured for different lengths of time in liquid (see Materials and Methods). This assay assumes that conidial inoculation from liquid medium without choline onto supplemented solid medium will not reset the clock phase. To confirm this, WT conidia (2 × 106 per milliliter) were cultured in liquid in constant light for 12 h, transferred to constant darkness (DD) to set the clock, and then, at intervals over the next day, 10-μl conidial suspensions were transferred onto race tubes. If inoculation resets the clock, the phase of the banding rhythm will be determined by the transfer from liquid to solid and will be different in each culture; if inoculation does not reset the phase, all culture phases will derive from the time of light to dark transfer and will be the same. We found that phases of all samples were close to the same, consistent with prior results (35) and confirming that conidial inoculation did not reset the clock (Fig. 2 A and B).
Fig. 2.
Normal circadian rhythms in liquid cultures not supplemented with choline. (A) Diagram of the conidia transfer assay (see Materials and Methods). If the clock is not reset by conidia transfer from liquid culture to race tube media, phases should be similar in all samples (light to dark reset). See Results for details. (B) Phase in WT conidia transfer assay. Phases of all WT cultures are similar regardless of the time of conidial transfer, indicating that liquid-to-solid medium transfer does not reset clock (error bars = 1 SD; n = 6). (C) Predicted phase differences in strains with longer (filled circles, period = 66 h), equal (open circles, period = 22 h), or shorter (solid inverted triangles, period = 11 h) period lengths in liquid culture compared with solid culture (see Results for details). (D) Conidia transfer assay of chol-1. Phase is reported from chol-1 cultured in liquid with no choline and inoculated onto race tubes supplemented with 100 μM choline (error bars = 1 SD; n = 6). Lack of significant phase differences among cultures transferred at different times suggests that the period under choline deficiency conditions is similar to that of fully choline-supplemented cultures.
Because we did not know the period of the oscillation in chol-1 strains in liquid culture without choline, we modeled the possible phase results using the formula shown in Materials and Methods (Fig. 2C). Obviously, if period length in liquid is close to that observed on solid medium with choline supplementation (≈22 h in period length), the phase of all cultures will be about the same, as was observed in the situation in WT strain (Fig. 2C). If the clock runs with a long period length rhythm in liquid (e.g., ≈60 h, like the CDO), a phase delay would be predicted in the samples inoculated later, because they spend more time in liquid with a slower clock. Alternatively, a phase advance is predicted if the liquid culture clock is running faster. Because csp-1 mutant strains do not release conidia well, a conidial liquid suspension was harvested from chol-1;csp-1+, inoculated into liquid without choline, germinated, and grown for 12 h in light before being transferred into DD. From this conidial suspension, samples cultured for different durations in liquid were inoculated onto race tubes supplemented with choline. After ≈8 days of growth the phases of banding rhythms were determined by regression to day 1; it is clear that all cultures have similar phases (Fig. 2D). This suggests that the circadian clock under choline starvation conditions runs with close to the same period as it does under choline supplementation on solid medium. Thus, the rhythm of the chol-1 strain in liquid medium with choline starvation appears to be regulated by the FWO instead of the CDO.
Normal Circadian Rhythmicity Under Conditions of Choline Starvation in a Race Tube Assay.
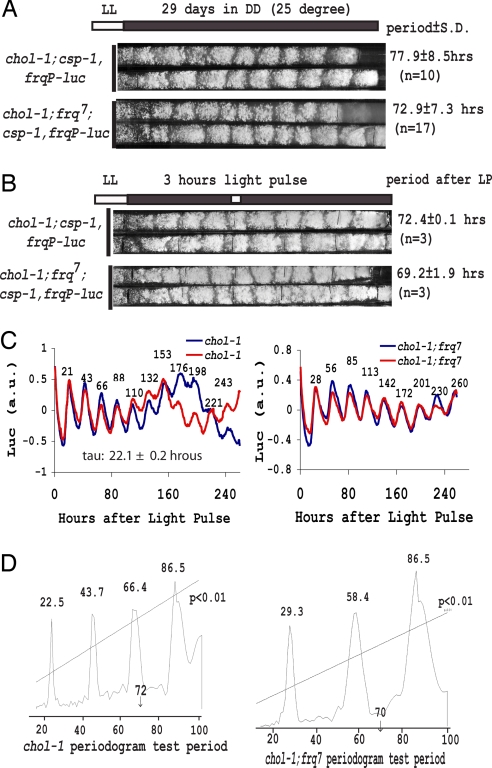
Although the results to this point had failed to shed light on any potential impact of choline starvation on the circadian system, there remained several strong caveats. First, it is difficult to know whether choline starvation in liquid culture is reached to the same degree as it is in cultures grown on solid medium lacking choline and expressing the CDO rhythm. Second, the CDO can sometimes take several days to become apparent. Third, and most importantly, we had not actually observed the FWO under conditions when we could also clearly follow the CDO. Because operation of the CDO is manifest only through its output in regulating growth and conidial development, we needed another assay for the FWO besides conidiation. To achieve this, we implemented a luciferase-based assay of WCC activity and circadian clock function. An optimized firefly luciferase reporter gene (36) under control of the frq promoter (frqP-luc) was used to follow the molecular rhythm of frq expression on solid medium in real time (see Materials and Methods). chol-1;csp-1,frqP-luc strains showed a long-period conidiation rhythm (≈78 h) on unsupplemented race tubes (Fig. 3A), consistent with previously published results for chol-1;csp-1 strains on minimal medium (30) and indicating that the strain was in full choline starvation and expressing the CDO.
Fig. 3.
frq allele-dependent luciferase rhythms with normal period lengths in a race tube assay under choline starvation conditions. (A) Characteristically elongated conidiation rhythms in chol-1;csp-1,frqP-luc and chol-1;csp-1,frqP-luc;frq7 strains. (B) Race tube assay containing 12.5 μM luciferin. Black lines were marked as reference points on the tube before strain growth. The 3 h of saturating light given after 292 h of growth (white bar) did not change the conidiation banding pattern but served to synchronize the FWOs in the culture. (C) Bioluminescence data collected from each of the whole race tubes and detrended to remove background reveals rhythms of ≈22-h period in WT strains and ≈29-h period in frq7 strains. The rhythms were sustained for >10 days under choline starvation whereas ≈70-h conidiation rhythms were still observed on the race tubes (B). Time values are marked on individual peaks of the detrended luciferase rhythms that show similar period and phase. (D) Periodogram analysis of the luciferase data using a window of 14–100 h shows a strong 22.5-h component in WT strain and a 29.3-h component in frq7 with their multiples but no appreciable contribution of rhythms with periods near 70 h.
The FWO was followed in the culture expressing the long-period CDO through the luciferase assay. To synchronize all of the FWO clocks, a 3-h light pulse was given to the race tube cultures after 292 h of growth, about halfway down to the tube (Fig. 3B). The long-period conidiation banding rhythm reflecting the CDO was not affected by the light pulse (compare Fig. 3 A and B) as predicted based on previous results (30). Initially, luciferase activity arising from the whole race tube was followed and is plotted in Fig. 3C. Consistent with normal FRQ oscillations in liquid culture without choline (Fig. 1B), frq promoter-regulated luciferase activity cycled with a normal period length (22.1 h in frq+ and 29.3 h in frq7) oscillation for >10 days [supporting information (SI) Fig. 5A, SI Table 1, and SI Movie 1]. The detrended data (in which the simple and linear increase in background level of luciferase was subtracted) clearly showed that luciferase rhythms from two independent chol-1 strains overlapped well in phase and in period, suggesting that it was accurately regulated by FWO (Fig. 3C) and despite the fact that the phases of the two CDO rhythms are not synchronous. Because the luc rhythm reports WCC activity through the frq promoter (13, 37), it is likely that many WCC-controlled genes (as well as ccgs, clock-controlled genes) are still under the control of the FWO even under choline deficiency conditions. Clearly, however, control of overt growth and development has passed from the FWO to the CDO in choline starvation conditions.
This visual assay, however, might not be sufficient to detect subtle interactions: we reasoned that, if the CDO was influencing the luc rhythms, it might be revealed within the data as a long-period trend having a period close to that of the conidiation rhythm. To evaluate this, we performed a periodogram analysis on the detrended luciferase data with period limits from 14 to 100 h. Within the period range tested, only the 22.5-h peak and its multiples were observed above the significance line (P < 0.01) (Fig. 3D). There were no frequency components having periods close to the period of conidiation rhythm (≈72 h), clearly suggesting that the CDO has minimal impact on the frqP-luc rhythm.
In a separate analysis, luciferase activity was followed from five consecutive CDO-controlled conidiation bands on a race tube after a 3-h light pulse (SI Fig. 5B). It should be noted that, at the time of the light pulse after which recording began, the growth front had not reached bands 3, 4, and 5, so for obvious reasons no luciferase activity is seen until the growth front passes those points. However, in all cases both raw data and detrended data showed normal circadian oscillations arising from individual CDO-controlled conidiation bands. Bands formed early showed a robust oscillation in the beginning and then slowly dampened away as nuclei migrated to the growth front and the regions become metabolically less active (38). Meanwhile, as the growth front moved ahead, oscillations appeared in newly formed tissue. As before, peaks of rhythms all appeared at close to the same time, which suggested that they were all controlled by the same FWO, and showed a slight phase gradient, with older regions tending to phase lag, as seen previously (39).
frq-Dependent and Temperature-Compensated Luciferase Rhythms Operate Independent of CDO-Controlled Development.
The CDO is neither temperature-compensated nor nutritionally compensated (30, 31) and shows a similar period length in frq+, frq7, and frq10 strains (31, 33, 40). In contrast, the observed luc rhythm should be temperature-compensated and frq-dependent because it was under control by the FWO. We constructed chol-1;frq7;csp-1,frqP-luc strains to test this hypothesis. The chol-1;frq7;csp-1,frqP-luc strains showed an ≈73-h conidiation rhythm under choline starvation (Fig. 3A), close to that seen for chol-1;csp-1,frqP-luc and consistent with the report that frq7 did not influence the conidiation rhythm period under choline deficiency (31). As before, a 3-h light pulse was given after 292 h in the dark, and the luciferase rhythm was subsequently monitored. The strain showed a luc rhythm of 28.74 ± 0.48 h period length (Fig. 3C and SI Table 1), typical for frq7 (41). Periodogram analysis on the raw luciferase data revealed a period of 29.3 h along with its multiples, with no indication of a frequency component having a period near 70 h (Fig. 3D). Again, the frqP-luc rhythm was controlled by the FWO with no apparent influence from the CDO.
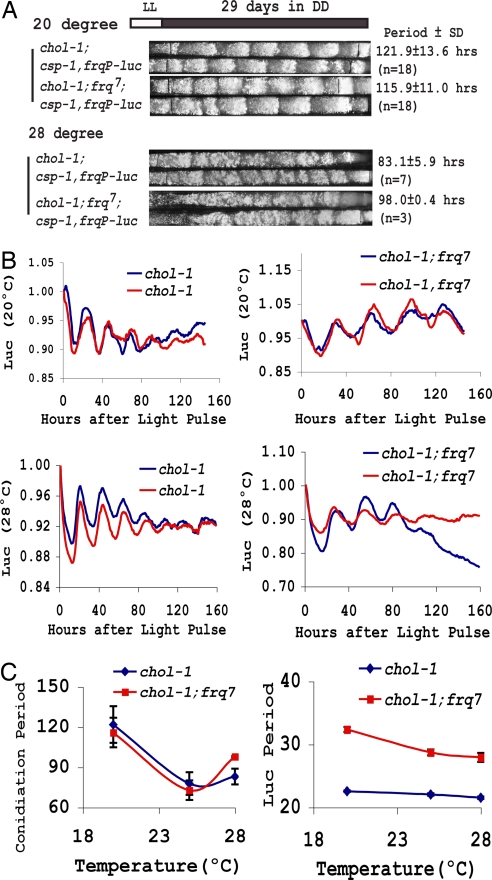
The FWO-controlled luc rhythm was also temperature-compensated. Luc rhythms examined at 20°C and 28°C (Fig. 4B) revealed a clear period difference between frq+ and frq7 at either temperature, confirming that the rhythm is frq-dependent. Comparison of period lengths in the same strain at different temperatures showed that the luc rhythm was temperature-compensated in chol-1, frq+ (chol-1;csp-1,frqP-luc) and slightly undercompensated in chol-1, frq7 (chol-1;frq7;csp-1,frqP-luc) (Fig. 4 B and C), as is appropriate for these frq alleles (42). We also monitored the CDO operation at two different temperatures: At 20°C the CDO showed a much longer period of ≈120 h (Fig. 4A), consistent with previous reported results (31), but at 28°C the CDO-driven banding was more frequent although less clear (Fig. 4A). These data, in conjunction with measurements of the CDO at 25°C (Fig. 3A), confirmed previous data (31) showing that the CDO rhythm is not temperature-compensated (Fig. 4C). In conclusion, these results indicate that the FWO and CDO coexist under choline deficiency conditions on solid media and that the FWO still maintains its circadian properties under these conditions. But we were unable to find evidence suggesting an influence of the CDO on the circadian properties of the FWO.
Fig. 4.
Temperature-compensated luciferase rhythms under conditions of choline depletion. (A) Race tube assays of chol-1 strains at 20°C and 28°C show the period of CDO-driven rhythmicity to be strongly temperature-dependent. (B) Luciferase rhythm at 20°C and 28°C. (C) FWO-driven luciferase rhythms are temperature-compensated in chol-1;csp-1,frqP-luc frq+ and undercompensated in chol-1;csp-1,frqP-luc;frq7, whereas the conidiation rhythm of CDO is not. Note the difference in scale (error bars = 1 SD; n = 3–17).
Discussion
Metabolic oscillators that do not require the transcription/translation-based FWO (FLOs) have been observed in Neurospora to influence many developmental and metabolic processes. The first FLO was identified two decades ago in frq-null strains where sometimes rhythmic banding would develop after several days' growth on race tubes (21, 25). Later, oscillations of gene expression, diacylglycerol levels, development, or nitrate reductase activity were found in either frq-null or wc-1-null strains (43–45). Recently, a FLO rhythm was discovered that requires WC-1 but not FRQ (46). In some cases the appearance of the FLO requires a specific genetic background (e.g., refs. 47–49) and in other cases specific nutritional supplements or the lack thereof (50–52). In all, at least nine different FLOs with periods that either are or can be in the circadian range are distinguishable based on some characteristic; although none of these has been shown to possess the full complement of circadian characteristics, most possess one or more of them, and nearly all FLOs have been suggested as possibly influencing the circadian system. The rationale for this is indeed attractive, because it seems plausible that metabolic oscillators operating in the same cytoplasm could or would in some way interact. Mechanisms of how those FLOs operate are still elusive, and the relationships between FLOs and the circadian FWO remain unknown, but the need for new tools and paradigms that can begin to bring order to and to describe a hierarchical relationship among this oscillatory menagerie has become apparent. The ability to directly monitor the circadian FWO in the absence of any of its outputs may be useful in this context.
The FLO revealed under choline deficiency conditions provided a manageable system in which to test this tool and approach, because this CDO is known to maintain its oscillation in the absence and presence of the FWO (31, 33). Here, we confirmed many of the basic characters of the CDO: It has a long period that is not influenced by the allelic state of frq in frq+ versus frq7, its phase is not consistent from culture to culture, it is not influenced by a short light treatment, and its period is not compensated between 20°C and 25°C.
We asked whether there was a connection between the CDO and the FWO and whether we could discover a role for the CDO in the circadian system. We began by following biochemical and physiological rhythms in liquid cultures starved for choline and found that under these conditions the FWO showed no apparent influence from the CDO. However, because little is known about the events associated with mutations leading to expression of the CDO, or how quickly CDO control is established after the start of choline starvation, there were clear caveats to these studies. The FWO, CDO, and most of the FLOs in Neurospora have been detected and observed only when one of these oscillators controls a rhythm in growth and development on race tubes. This precludes the observation of two or more rhythms at the same time. To circumvent this problem, we adopted a luciferase based assay of the FWO function. Using this assay we found that the circadian FWO functions without apparent alteration under conditions of obvious choline starvation. The FWO operates in parallel with the CDO in the same tissue at the same time but is apparently unaffected by the CDO (Fig. 3 and SI Movie 1). Although these studies identified no apparent role for the CDO in the circadian system, it is possible that additional work could detect such a role.
We have not, of course, determined whether there is any physiological relevance to the noncircadian control by the CDO. The extreme variability of its period and phase, and the lack of correspondence between CDO periods and known environmental periodicities, hamper efforts to define significance. The highly episodic nature of CDO-controlled growth (reviewed in refs. 4, 10, and 24) distinguishes it from growth controlled by the circadian FWO and is suggestive of a relaxation oscillator perhaps timed by the slow accumulation of the metabolites that allow growth of the leaky chol-1 auxotroph. Even the signals eliciting conidiation (by the circadian FWO) or temporarily overcoming a limitation (by the CDO) would be different; the nature of the signals promoting conidiation may be subtle and complex (53). At present, however, the most straightforward interpretation of the data presented here is that the CDO represents an alternative oscillatory state that can bypass circadian control of conidiation but appears in no way connected to the circadian system.
More generally, this study shows that, by using the luc rhythms as a reporter of the FWO circadian feedback loop, it will be possible to extend these studies to other noncircadian oscillators or even FLOs in Neurospora and thereby to dissect the hierarchy of controls and interacting oscillators within the circadian system.
Materials and Methods
Strains.
All strains used carry the ras-1bd mutation, which promotes expression of the conidiation rhythm (53). PL32-54 (bd;csp-1;chol-1) was a kind gift of P. L. Lakin-Thomas (York University, Toronto). In frqP-luc strains, a codon-optimized firefly luciferase gene under the control of the frq promoter was integrated into the his-3 locus (36). The strain chol-1;csp-1,frqP-luc was a progeny from a cross between frqP-luc and PL32-54. The strain chol-1;csp-1, frqP-luc;frq7 was a progeny from a cross between chol-1;csp-1,frqP-luc and frq7. Strains chol-1;csp-1+ were cross-progeny from PL32-54 and WT strains.
Culture Conditions.
All studies were performed at 25°C, except the specified 20°C or 28°C assays. Vogel's medium (pH 4.5) with 0.4% sucrose and 1.5% agar was used for race tube assays. Choline chloride from Sigma was used as a choline supplement. Period and phase values from race tube assays were measured by using the Chrono program (54) in choline-supplemented cultures. In chol-1 mutant strains not supplemented with choline, growth rate and distance between conidiation bands were measured and period was calculated by dividing the banding distance by the growth rate.
Vogel's salts and 2% glucose were used for liquid culture (55). For time-course experiments, mycelial discs (8 mm in diameter for choline supplement growth and 20 mm in diameter for no choline supplementation) were inoculated into flasks containing liquid media.
For measurement of luciferase activity, solid race tube medium was supplemented with 12.5 μM luciferin. Light arising from in vivo luciferase activity was collected for 10 min every hour with a VersArray 1300 B/LN liquid nitrogen-cooled CCD camera from Princeton Instruments, controlled by aWinView/32 software from Roper Scientific. A Percival incubator was modified to fit the lens of the VersArray system. All luciferase rhythm data were analyzed by BRASS (Biological Rhythm Analysis Software System; www.amillar.org), in which estimates of periodicity are determined by FFT-NLLS (fast Fourier transform–nonlinear least squares) (56). The detrended rhythms as plotted were processed by using the “detrend” function in the Matlab software package. The periodograms were processed by using the Chrono program (54).
Protein Assays.
Mycelial tissues were harvested from cultures in flasks and frozen in liquid nitrogen. The frozen tissues were ground in liquid nitrogen by using a mortar and pestle and processed for Western blot analysis as described (55). Densitometry analysis was performed by using the NIH Image program.
Conidia Transfer Assay.
In the liquid-to-solid culture transfer assay (Fig. 2A), conidia from chol-1 strains grown on Vogel's medium supplemented with 100 μM choline were harvested, washed three times with 150 ml of liquid media (Vogel's salts, 2% glucose, no choline), and concentrated to 2 × 106 per milliliter, and 10 μl of the conidia suspension was inoculated onto race tubes supplemented with 100 μM choline as a phase control. In parallel, 10 ml of the suspension (2 × 106 per milliliter) was cultured in light, and after 12 h both the liquid culture and race tubes were moved into DD to set the phase of the clock. Ten times over the subsequent 20 h, 10 μl of the conidia suspension was removed from the liquid culture and inoculated onto choline-supplemented (100 μM) race tubes. The tubes were kept in DD for 8 days, and growth fronts were marked daily (34). Phase information for each race tube was obtained by using Chrono (54).
To calculate the inferred period length of the clock in liquid culture (Fig. 2), we assumed that, if the clock period length in liquid versus solid medium was different, then the phase observed on a series of race tubes after inoculation would be different, reflecting the different durations of culture in liquid. (Fig. 2B confirms that conidial inoculation itself does not reset the clock.) Based on this, the following formula calculates the phase a culture would have when transferred from liquid culture: Dp = I/Pl × Ps − I, where Dp is the phase difference of two samples, I is the time interval between inoculation of two samples, Pl is the period length in liquid culture, and Ps is the period length on solid medium.
Supplementary Material
Acknowledgments
We thank Van Gooch and Arun Mehra for help with implementation of the luciferase assay. This work was supported by National Institutes of Health Grants GM83336 (to J.C.D. and J.J.L.) and GM34985 (to J.C.D.). L.F.L. is a Pew Latin American Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706631104/DC1.
References
- 1.Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Proc Natl Acad Sci USA. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hastings JW, Sweeney BM. Proc Natl Acad Sci USA. 1957;43:804–811. doi: 10.1073/pnas.43.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sargent ML, Briggs WR, Woodward DO. Plant Physiol. 1966;41:1343–1349. doi: 10.1104/pp.41.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlap JC. Annu Rev Physiol. 1993;55:683–728. doi: 10.1146/annurev.ph.55.030193.003343. [DOI] [PubMed] [Google Scholar]
- 5.Lakin-Thomas PL, Cote GG, Brody S. Crit Rev Microbiol. 1990;17:365–416. doi: 10.3109/10408419009114762. [DOI] [PubMed] [Google Scholar]
- 6.Feldman JF, Hoyle MN. Genetics. 1973;75:605–613. doi: 10.1093/genetics/75.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronson BD, Johnson KA, Loros JJ, Dunlap JC. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- 8.McClung CR, Fox BA, Dunlap JC. Nature. 1989;339:558–562. doi: 10.1038/339558a0. [DOI] [PubMed] [Google Scholar]
- 9.Brunner M, Schafmeier T. Genes Dev. 2006;20:1061–1074. doi: 10.1101/gad.1410406. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap JC, Loros JJ. J Biol Rhythms. 2004;19:414–424. doi: 10.1177/0748730404269116. [DOI] [PubMed] [Google Scholar]
- 11.Dunlap JC, Loros JJ. Curr Opin Microbiol. 2006;9:579–587. doi: 10.1016/j.mib.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Bell-Pedersen D. Eukaryotic Cell. 2006;5:1184–1193. doi: 10.1128/EC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosthwaite SK, Dunlap JC, Loros JJ. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- 14.Cheng P, He Q, He Q, Wang L, Liu Y. Genes Dev. 2005;19:234–241. doi: 10.1101/gad.1266805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo C, Loros JJ, Dunlap JC. EMBO J. 1998;17:1228–1235. doi: 10.1093/emboj/17.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Q, Cha J, He Q, Lee HC, Yang Y, Liu Y. Genes Dev. 2006;20:2552–2565. doi: 10.1101/gad.1463506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafmeier T, Haase A, Kaldi K, Scholz J, Fuchs M, Brunner M. Cell. 2005;122:235–246. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 18.He Q, Cheng P, Yang Y, He Q, Yu H, Liu Y. EMBO J. 2003;22:4421–4430. doi: 10.1093/emboj/cdg425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schafmeier T, Kaldi K, Diernfellner A, Mohr C, Brunner M. Genes Dev. 2006;20:297–306. doi: 10.1101/gad.360906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunlap JC. J Biol Chem. 2006;281:28489–28493. doi: 10.1074/jbc.R600018200. [DOI] [PubMed] [Google Scholar]
- 21.Aronson BD, Johnson KA, Dunlap JC. Proc Natl Acad Sci USA. 1994;91:7683–7687. doi: 10.1073/pnas.91.16.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collett MA, Garceau N, Dunlap JC, Loros JJ. Genetics. 2002;160:149–158. doi: 10.1093/genetics/160.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, Dunlap JC, Loros JJ. Genetics. 2003;163:103–114. doi: 10.1093/genetics/163.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakin-Thomas PL, Brody S. Annu Rev Microbiol. 2004;58:489–519. doi: 10.1146/annurev.micro.58.030603.123744. [DOI] [PubMed] [Google Scholar]
- 25.Loros JJ, Feldman JF. J Biol Rhythms. 1986;1:187–198. doi: 10.1177/074873048600100302. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki H, Dunlap JC. Curr Opin Microbiol. 2000;3:189–196. doi: 10.1016/s1369-5274(00)00074-6. [DOI] [PubMed] [Google Scholar]
- 27.Bell-Pedersen D, Crosthwaite SK, Lakin-Thomas PL, Merrow M, Okland M. Philos Trans R Soc London B. 2001;356:1697–1709. doi: 10.1098/rstb.2001.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattern DL, Forman LR, Brody S. Proc Natl Acad Sci USA. 1982;79:825–829. doi: 10.1073/pnas.79.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarborough GA, Nyc JF. Biochim Biophys Acta. 1967;146:111–119. doi: 10.1016/0005-2744(67)90077-0. [DOI] [PubMed] [Google Scholar]
- 30.Lakin-Thomas PL. Biol Rhythm Res. 1996;27:12–30. [Google Scholar]
- 31.Lakin-Thomas PL. J Biol Rhythms. 1998;13:268–277. doi: 10.1177/074873098129000101. [DOI] [PubMed] [Google Scholar]
- 32.Ruoff P, Slewa I. Chronobiol Int. 2002;19:517–529. doi: 10.1081/cbi-120004222. [DOI] [PubMed] [Google Scholar]
- 33.Lakin-Thomas PL, Brody S. Proc Natl Acad Sci USA. 2000;97:256–261. doi: 10.1073/pnas.97.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunlap JC, Loros JJ. Methods Enzymol. 2005;393:3–22. doi: 10.1016/S0076-6879(05)93001-2. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima H, Perlman J, Feldman JF. Am J Physiol. 1981;241:R31–R35. doi: 10.1152/ajpregu.1981.241.1.R31. [DOI] [PubMed] [Google Scholar]
- 36.Gooch V, Mehra A, Larrondo LF, Fox J, Touroutoutoudis M, Loros JJ, Dunlap JC. Eukaryotic Cell. 2008 doi: 10.1128/EC.00257-07. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Froehlich AC, Liu Y, Loros JJ, Dunlap JC. Science. 2002;297:815–819. doi: 10.1126/science.1073681. [DOI] [PubMed] [Google Scholar]
- 38.Castro-Longoria E, Brody S, Bartnicki-Garcia S. Fungal Genet Biol. 2007;44:672–681. doi: 10.1016/j.fgb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Dharmananda S, Feldman JF. Plant Physiol. 1979;63:1049–1054. doi: 10.1104/pp.63.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakin-Thomas PL. Trends Genet. 2000;16:135–142. doi: 10.1016/s0168-9525(99)01945-9. [DOI] [PubMed] [Google Scholar]
- 41.Gardner GF, Feldman JF. Genetics. 1980;96:877–886. doi: 10.1093/genetics/96.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner GF, Feldman JF. Plant Physiol. 1981;68:1244–1248. doi: 10.1104/pp.68.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen MK, Falkeid G, Loros JJ, Dunlap JC, Lillo C, Ruoff P. J Biol Rhythms. 2004;19:280–286. doi: 10.1177/0748730404265532. [DOI] [PubMed] [Google Scholar]
- 44.Correa A, Lewis ZA, Greene AV, March IJ, Gomer RH, Bell-Pedersen D. Proc Natl Acad Sci USA. 2003;100:13597–13602. doi: 10.1073/pnas.2233734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsdale M, Lakin-Thomas PL. J Biol Chem. 2000;275:27541–27550. doi: 10.1074/jbc.M002911200. [DOI] [PubMed] [Google Scholar]
- 46.de Paula RM, Lewis ZA, Greene AV, Seo KS, Morgan LW, Vitalini MW, Bennett L, Gomer RH, Bell-Pedersen D. J Biol Rhythms. 2006;21:159–168. doi: 10.1177/0748730406288338. [DOI] [PubMed] [Google Scholar]
- 47.He Q, Cheng P, He Q, Liu Y. Genes Dev. 2005;19:1518–1531. doi: 10.1101/gad.1322205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattern DL, Forman LR, Brody S. Proc Natl Acad Sci USA. 1982;79:825–829. doi: 10.1073/pnas.79.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lombardi L, Schneider K, Tsukamoto M, Brody S. Genetics. 2007;175:1175–1183. doi: 10.1534/genetics.106.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brody S, Martins SA. J Bacteriol. 1979;137:912–915. doi: 10.1128/jb.137.2.912-915.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lakin-Thomas PL, Brody S. Proc Natl Acad Sci USA. 2000;97:256–261. doi: 10.1073/pnas.97.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Granshaw T, Tsukamoto M, Brody S. J Biol Rhythms. 2003;18:287–296. doi: 10.1177/0748730403255934. [DOI] [PubMed] [Google Scholar]
- 53.Belden WJ, Larrondo LF, Froehlich AC, Shi M, Chen CH, Loros JJ, Dunlap JC. Genes Dev. 2007;21:1494–1505. doi: 10.1101/gad.1551707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roenneberg T, Taylor W. Methods Enzymol. 2000;305:104–119. doi: 10.1016/s0076-6879(00)05481-1. [DOI] [PubMed] [Google Scholar]
- 55.Garceau NY, Liu Y, Loros JJ, Dunlap JC. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- 56.Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA. J Biol Rhythms. 1997;12:204–217. doi: 10.1177/074873049701200302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.