Figure 4. Energetic contributions of the paratope residues of the affinity-matured VHH probed with Ala-scanning mutagenesis.
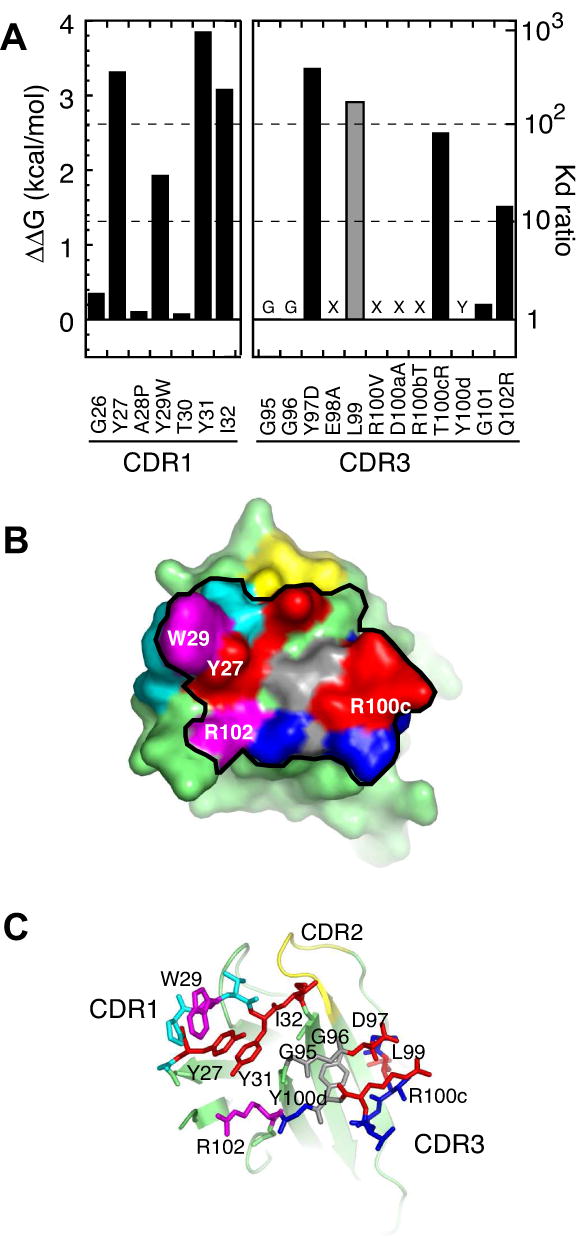
(A) Effects of Ala substitution on antigen binding expressed in terms of ΔΔG (the left axis) and the ratio of Kd (= Kdwild-type/Kdmutant; the right axis). Positions 95, 96, 98, 100-100b and 100d were not tested because (i) Gly residue is critical (labeled with “G”), (ii) no sequence convergence was found in library screening (labeled with “X”) or (iii) the Tyr is involved in scaffolding (labeled with “Y”). The value for L99, a noncontacting residue, is shown in gray. (B) The surface representation of the affinity-matured VHH. The black line encloses residues within 5 Å of antigen atoms. Red and cyan surfaces are for residues where Ala substitution causes >100 fold and 10-100 fold increase in Kd, respectively. Gray surfaces indicate critical residues that were not tested with Ala scanning. The remaining CDR1 and CDR3 residues are shown in light blue and dark blue, respectively. The four residues that expand the hot spot (W29, D97, R100c and R102) are labeled. (C) shows CDR1 and CDR3 residues as sticks and the VHH scaffold as a cartoon model.
