Abstract
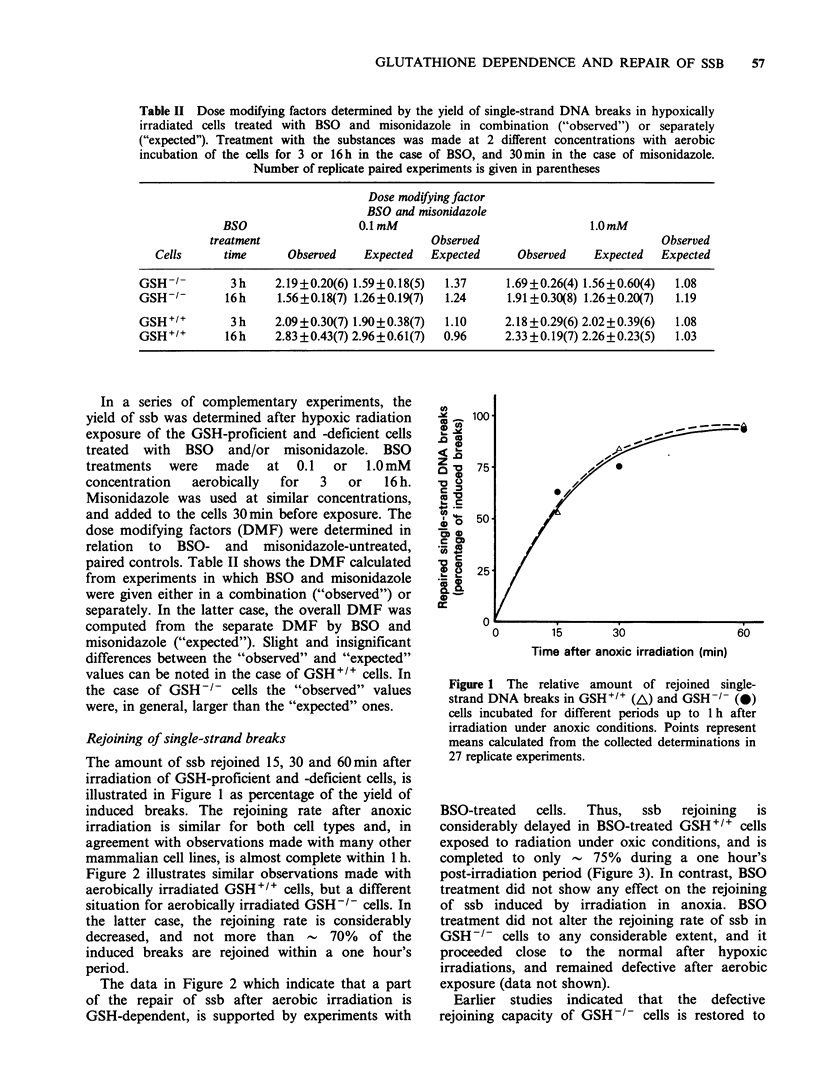
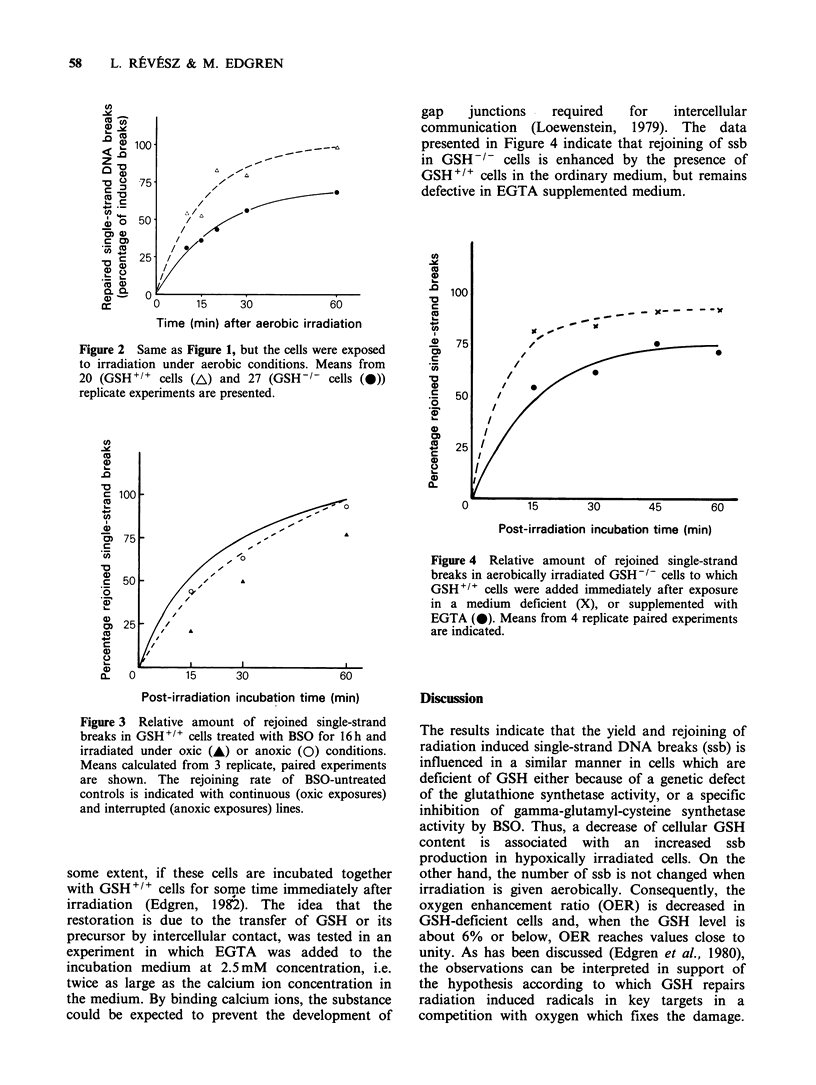
The yield and rejoining of single-strand DNA breaks (ssb) was investigated after irradiation of cells which were deficient in glutathione (GSH) either due to a genetic defect of their GSH synthetase activity, or inhibition of gamma-glutamylcysteine synthetase activity by DL-buthionine-SR-sulfoximine (BSO). The results were concordant in indicating that decreased cellular GSH content is associated with an increased yield of ssb after anoxic, but not after aerobic radiation exposures. Rejoining of ssb was delayed and incomplete during a one hour's incubation period after oxic, but not after anoxic exposure of GSH-deficient cells. The defective rejoining capacity of these cells was restituted to nearly normal by the admixture of GSH-proficient cells in the incubation medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dethmers J. K., Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgren M., Larsson A., Nilsson K., Révész L., Scott O. C. Lack of oxygen effect in glutathione-deficient human cells in culture. Int J Radiat Biol Relat Stud Phys Chem Med. 1980 Mar;37(3):299–306. doi: 10.1080/09553008014550341. [DOI] [PubMed] [Google Scholar]
- Edgren M., Révész L., Larsson A. Induction and repair of single-strand DNA breaks after X-irradiation of human fibroblasts deficient in glutathione. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Oct;40(4):355–363. doi: 10.1080/09553008114551311. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Modig H. G. Interaction of cysteamine with the thiol and disulphide groups in deoxyribonucleoproteins. Biochem Pharmacol. 1973 Jul 1;22(13):1623–1631. doi: 10.1016/0006-2952(73)90029-4. [DOI] [PubMed] [Google Scholar]
- REVESZ L., BERGSTRAND H., MODIG H. Intrinsic nonprotein sulphydryl levels and cellular radiosensitivity. Nature. 1963 Jun 29;198:1275–1277. doi: 10.1038/1981275a0. [DOI] [PubMed] [Google Scholar]
- Sinclair W. K. Protection by cysteamine against lethal x-ray damage during the cell cycle of Chinese hamster cells. Radiat Res. 1969 Jul;39(1):135–154. [PubMed] [Google Scholar]
- Sjöberg L., Eriksen T. E., Révész L. The reaction of the hydroxyl radical with glutathione in neutral and alkaline aqueous solution. Radiat Res. 1982 Feb;89(2):255–263. [PubMed] [Google Scholar]


