Figure 4.
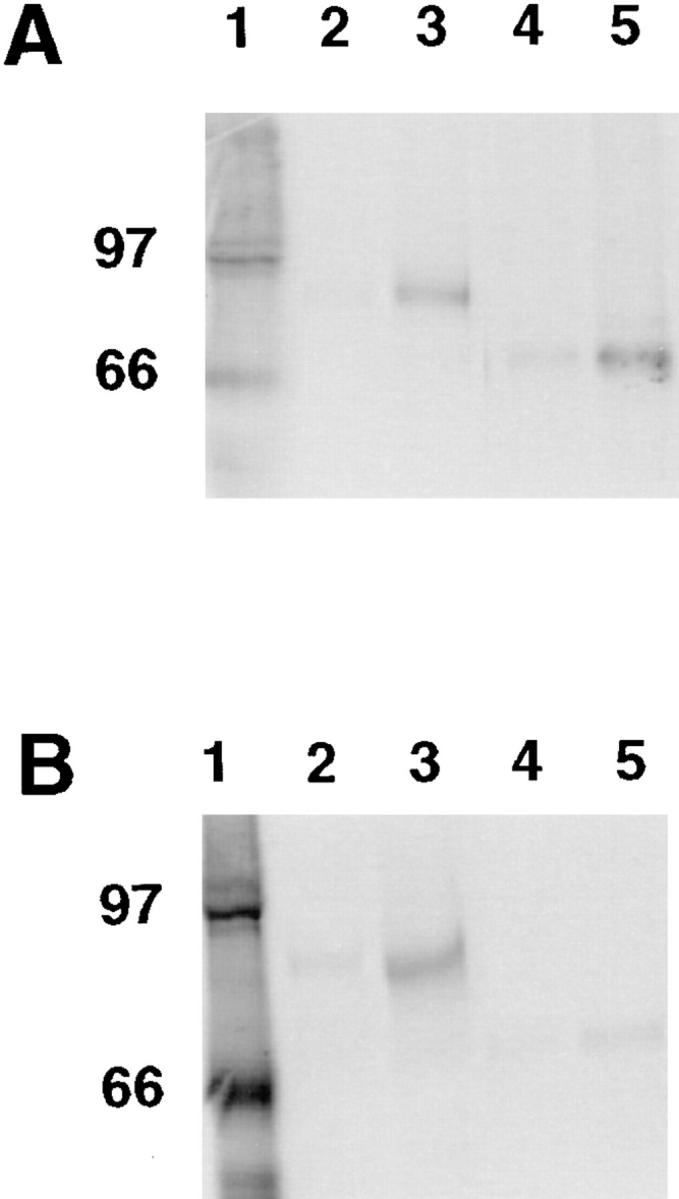
Analysis of dystrobrevin– dystrophin and dystrobrevin–utrophin interactions by coimmunoprecipitation of in vitro–translated proteins. α-Dystrobrevin-1 or -2 was synthesized in the presence of 14C-leucine; utrophin and dystrophin were synthesized as non-radioactive forms. In vitro–translated proteins were incubated together, and then precipitated with a specific antibody in the presence of protein G–Sepharose. After centrifugation and washing, bound proteins were eluted in sample buffer and analyzed by SDS-PAGE and autoradiography. (A) Lane 1, [14C]methylated molecular weight standards. Lane 2, control in which radiolabeled α-dystrobrevin-1 was incubated with dystrophin, but anti-dystrophin was excluded from the immunoprecipitation. Lane 3, radiolabeled α-dystrobrevin-1 was incubated with dystrophin and complexes were immunoprecipitated with anti-dystrophin. Lane 4, control in which radiolabeled α-dystrobrevin-2 was incubated with dystrophin, but anti-dystrophin was excluded from the immunoprecipitation. Lane 5, radiolabeled α-dystrobrevin-2 was incubated with dystrophin and complexes were immunoprecipitated with anti-dystrophin. (B) Lane 1, [14C]methylated molecular weight standards. Lane 2, control in which radiolabeled α-dystrobrevin-1 was incubated with utrophin, but anti-utrophin was excluded from the immunoprecipitation. Lane 3, radiolabeled α-dystrobrevin-1 was incubated with utrophin and complexes were immunoprecipitated with anti-utrophin. Lane 4, control in which radiolabeled α-dystrobrevin-2 was incubated with utrophin, but anti-utrophin was excluded from the immunoprecipitation. Lane 5, radiolabeled α-dystrobrevin-2 was incubated with utrophin and complexes were immunoprecipitated with anti-utrophin. A background of nonspecific aggregation and precipitation of α-dystrobrevin-1 and -2 was seen, regardless of whether a specific antibody was used.
