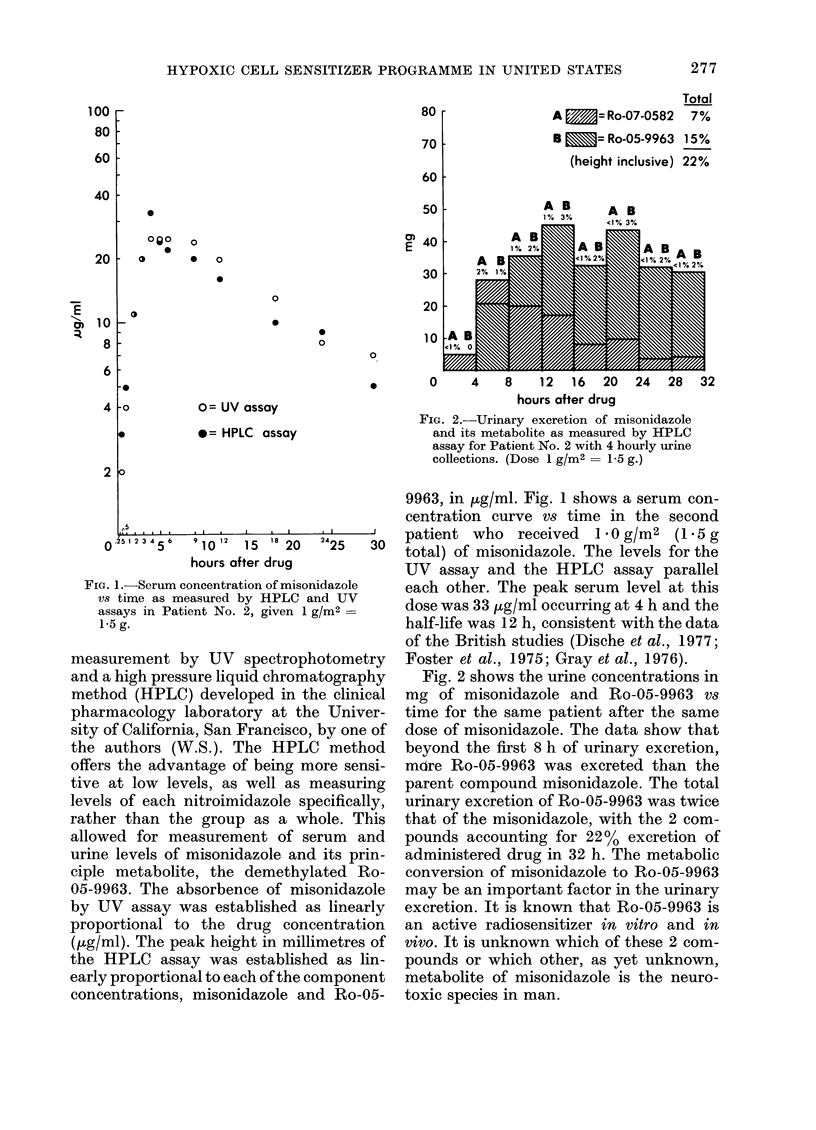
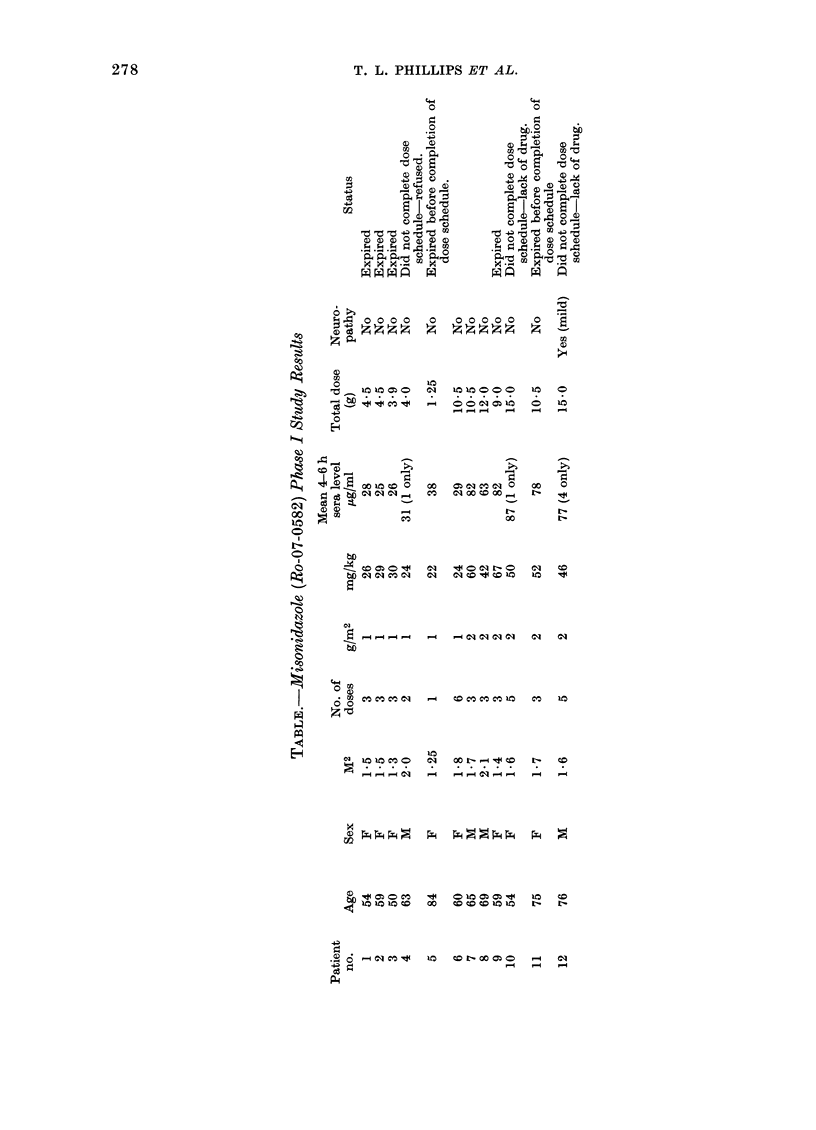
Abstract
The initial results of a Phase I evaluation of misonidazole in the U.S.A. are described, as well as the U.S. National Cancer Institute programme for radiosensitizer development. A total of 12 patients have been given 1--6 doses of 1--2 g/m2. Serum levels ranged from 25--87 microgram/ml at 4--6 h. One patient has developed mild peripheral neuropathy. Urinary excretion was chiefly of a demethylated metabolite as measured by HPLC assay.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dische S., Saunders M. I., Lee M. E., Adams G. E., Flockhart I. R. Clinical testing of the radiosensitizer Ro 07-0582: experience with multiple doses. Br J Cancer. 1977 May;35(5):567–579. doi: 10.1038/bjc.1977.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. L., Flockhart I. R., Dische S., Gray A., Lenox-Smith I., Smithen C. E. Serum concentration measurements in man of the radiosensitizer Ro-07-0582: some preliminary results. Br J Cancer. 1975 Jun;31(6):679–683. doi: 10.1038/bjc.1975.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A. J., Dische S., Adams G. E., Flockhart I. R., Foster J. L. Clinical testing of the radiosensitiser Ro-07-0582. I. Dose tolerance, serum and tumour concentrations. Clin Radiol. 1976 Apr;27(2):151–157. doi: 10.1016/s0009-9260(76)80137-7. [DOI] [PubMed] [Google Scholar]


