Figure 1.
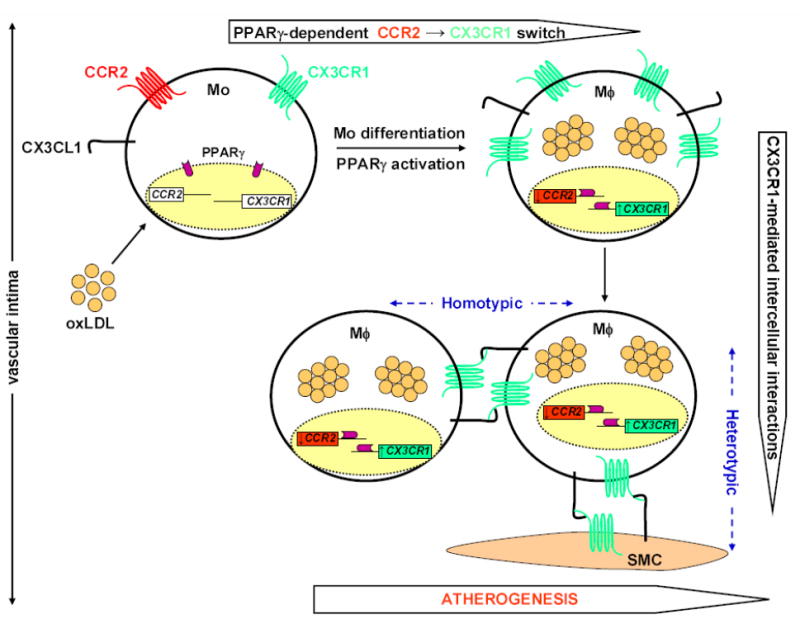
A pro-adhesive PPARγ-mediated chemokine receptor switch, CCR2→CX3CR1, in atherosclerosis. LDL diffuses from the blood into the vascular intima, where it undergoes oxidative modifications forming randomly distributed extracellular pools of oxLDL and its stable linoleic acid metabolites. Circulating blood monocytes expressing both CCR2 and CX3CR1 invade inflamed arteries in response to pro-inflammatory cytokines and chemoattractants produced by vascular endothelium through a multi-step process that includes selectin-mediated rolling, integrin-firm arrest, spreading and diapedesis. After entering the subendothelial space, the atheromatous microenvironment rich in oxLDL stimulates monocyte differentiation into foamy macrophages that are the predominant cell population in early atherosclerotic lesions. Furthermore, ingestion of oxLDL by monocytes activates the transcription factor PPARγ, which not only participates in maturation of monocytes to macrophages, but also promotes simultaneous downregulation of CCR2 and upregulation of the adhesion chemokine receptor CX3CR1 expression, thus promoting cessation of macrophage migration, and their capture and retention in the plaque. Furthermore, since CX3CR1 and its ligand CX3CL1 are expressed by various cell types in lesions, this pro-adhesive CCR2→CX3CR1 switch may support formation of numerous heterotypic and homotypic intercellular interactions maintained by a pro-atherogenic CX3CL1-CX3CR1 axis that may contribute towards organization of cells in plaque. Mo, monocyte; Mϕ, foamy macrophage; oxLDL, oxidized LDL; SMC, smooth muscle cell.
