Abstract
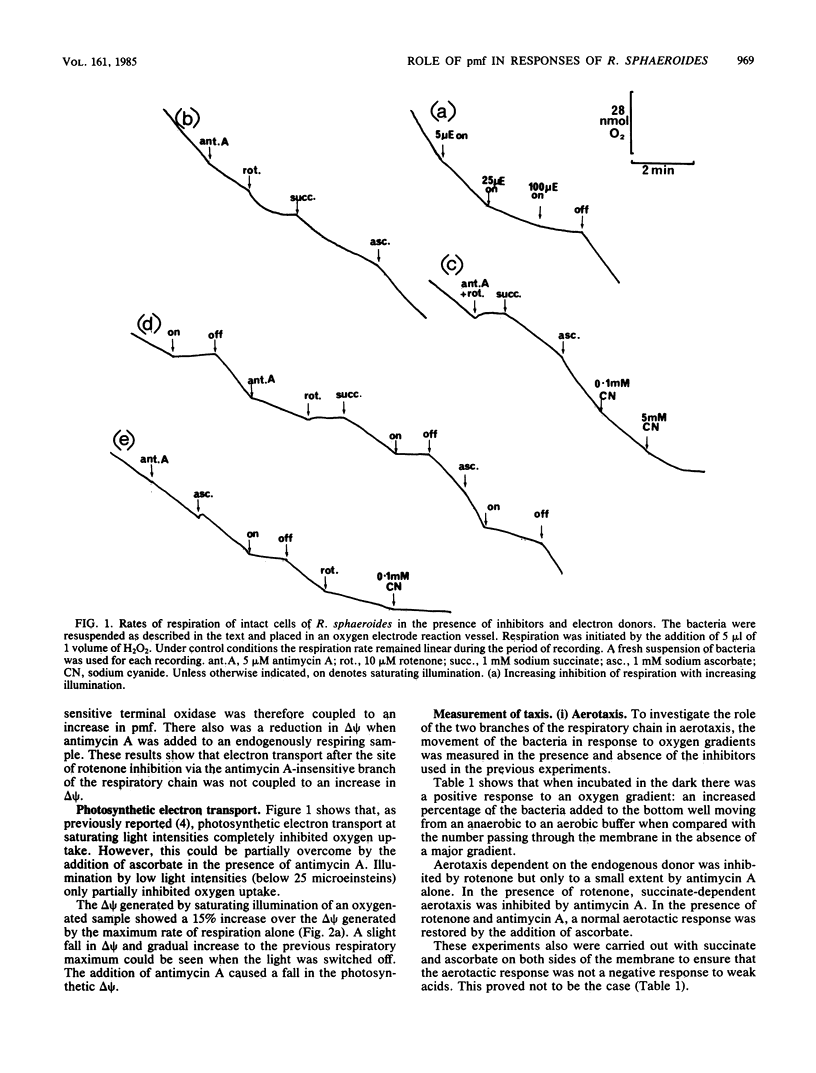
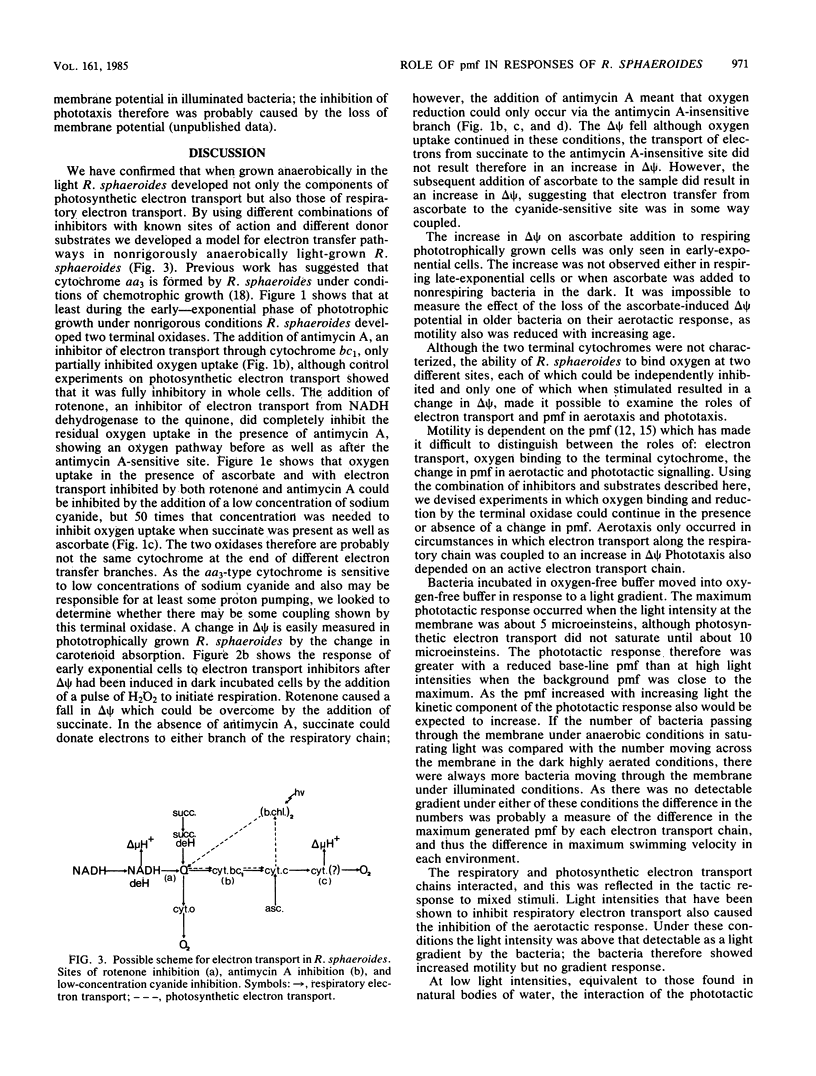
Rhodopseudomonas sphaeroides grown under nonrigorous anaerobic conditions in the light developed components of a branched respiratory electron transfer chain, and a photosynthetic electron transfer chain. Both respiratory pathways were sensitive to rotenone and high concentrations of cyanide, but oxygen uptake was only partially inhibited by the addition of low concentrations of cyanide or antimycin A. When incubated anaerobically in the dark, R. sphaeroides responded positively to an oxygen gradient in the absence of rotenone. In the presence of rotenone, aerotaxis only occurred when the antimycin A-sensitive branch of the pathway was functioning, although both branches still reduced oxygen. Although there was electron movement along the respiratory chain, aerotaxis only occurred in response to a change in proton motive force. When incubated anaerobically in the light, the movement of R. sphaeroides up a light gradient depended on photosynthetic electron transport. When incubated aerobically, high-intensity actinic illumination inhibited oxygen uptake and aerotaxis. In a low-intensity light gradient the phototactic response was inhibited by oxygen. These results are discussed in relation to the interaction of the electron transfer chains and their roles in controlling tactic responses in R. sphaeroides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage J. P., Evans M. C. Membrane potential changes during chemotaxis of Rhodopseudomonas sphaeroides. FEBS Lett. 1979 Jun 1;102(1):143–146. doi: 10.1016/0014-5793(79)80946-1. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. The induced synthesis of catalase in Rhodopseudomonas spheroides. Biochim Biophys Acta. 1960 Jan 29;37:503–512. doi: 10.1016/0006-3002(60)90507-2. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Jackson J. B. The measurement of membrane potential during photosynthesis and during respiration in intact cells of Rhodopseudomonas capsulata by both electrochromism and by permeant ion redistribution. Biochem J. 1981 Nov 15;200(2):389–397. doi: 10.1042/bj2000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton N. P., Clark A. J., Jackson J. B. Interaction between the respiratory and photosynthetic electron transport chains of intact cells of Rhodopseudomonas capsulata mediated by membrane potential. Eur J Biochem. 1983 Feb 15;130(3):581–587. doi: 10.1111/j.1432-1033.1983.tb07189.x. [DOI] [PubMed] [Google Scholar]
- Eisenbach M., Margolin Y., Ciobotariu A., Rottenberg H. Distinction between changes in membrane potential and surface charge upon chemotactic stimulation of Escherichia coli. Biophys J. 1984 Feb;45(2):463–467. doi: 10.1016/S0006-3495(84)84169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulbourne E. A., Jr, Greenberg E. P. Chemotaxis of Spirochaeta aurantia: involvement of membrane potential in chemosensory signal transduction. J Bacteriol. 1981 Dec;148(3):837–844. doi: 10.1128/jb.148.3.837-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980 Apr 15;138(3):599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Laszlo D. J., Taylor B. L. Aerotaxis in Salmonella typhimurium: role of electron transport. J Bacteriol. 1981 Feb;145(2):990–1001. doi: 10.1128/jb.145.2.990-1001.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J. E., Manson M. D., Berg H. C. Signal processing times in bacterial chemotaxis. Nature. 1982 Apr 29;296(5860):855–857. doi: 10.1038/296855a0. [DOI] [PubMed] [Google Scholar]
- Taylor B. L. Role of proton motive force in sensory transduction in bacteria. Annu Rev Microbiol. 1983;37:551–573. doi: 10.1146/annurev.mi.37.100183.003003. [DOI] [PubMed] [Google Scholar]
- Ward J. A., Hunter C. N., Jones O. T. Changes in the cytochrome composition of Rhodopseudomonas sphaeroides grown aerobically, photosynthetically and on dimethyl sulphoxide. Biochem J. 1983 Jun 15;212(3):783–790. doi: 10.1042/bj2120783. [DOI] [PMC free article] [PubMed] [Google Scholar]


