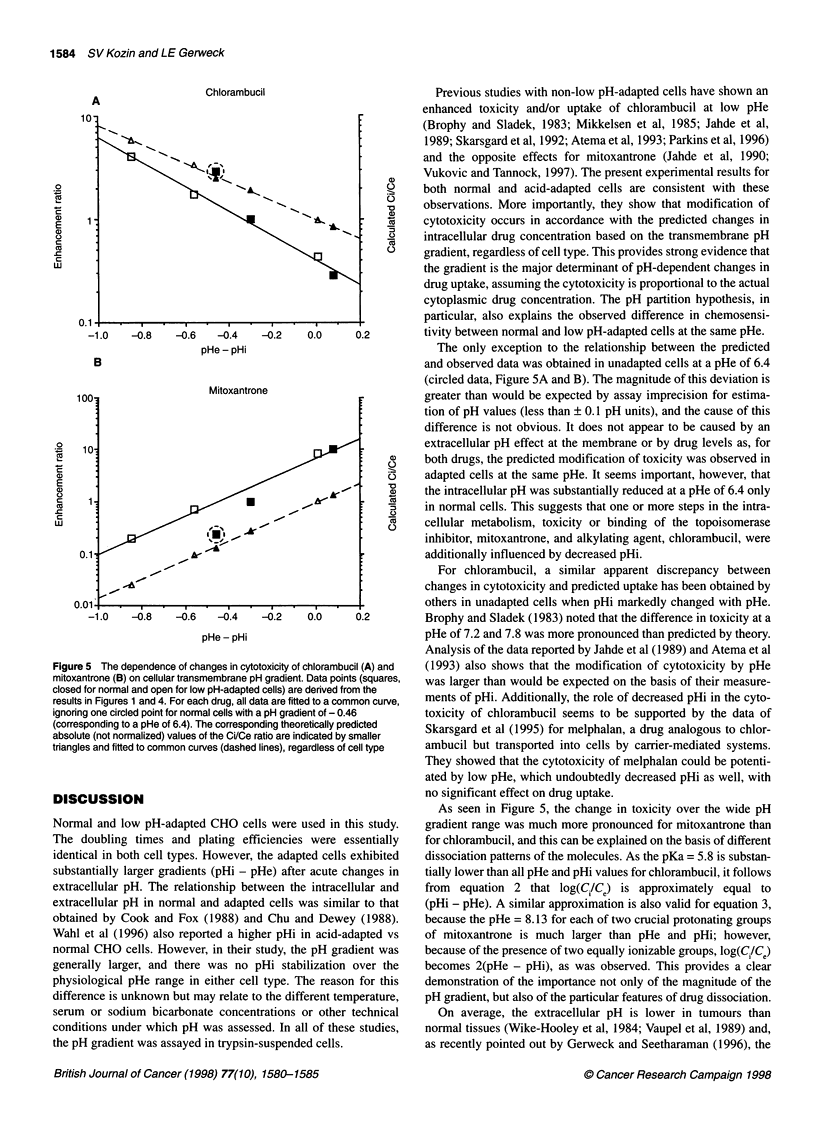
Abstract
Theory suggests that the transmembrane pH gradient may be a major determinant of the distribution of lipophilic weak electrolytes across the cell membrane. The present study evaluates the extent to which this factor contributes to pH-dependent changes in the cytotoxicity of two such chemotherapeutic drugs: chlorambucil and mitoxantrone. Experiments were performed with two cell types of the same origin but exhibiting different pH gradients at the same extracellular pH (pHe): CHO cells cultured under normal physiological conditions (pH 7.4) and acid-adapted cells obtained by culturing under low pH conditions (6.8). Over the pHe range examined (6.0-7.6), the difference between intracellular pH (pHi) and pHe increased with decreasing pHe. Acid-adapted cells were more resistant to acute changes in pHi than normal cells, resulting in substantially larger gradients in these cells. Drug cell survival curves were performed at pHe values of 6.4, 6.8 and 7.4. The cytotoxicity of chlorambucil, a weak acid, increased with decreasing pHe, and low pH-adapted cells were more sensitive than normal cells at the same pHe. In contrast, for the weak base, mitoxantrone, cytotoxicity increased with pHe and was more pronounced in normal cells. As predicted by the theory, the cytotoxicity of both drugs changed exponentially as a function of the pH gradient, regardless of cell type. For mitoxantrone, the rate of such change in cytotoxicity with the gradient was approximately two times greater than for chlorambucil. This difference is probably due to the presence of two equally ionizable crucial groups on mitoxantrone vs one group on chlorambucil. It is concluded that the cellular pH gradient plays a major role in the pH-dependent modulation of cytotoxicity in these weak electrolytes. The data obtained also suggest that a pronounced differential cytotoxicity may be expected in vivo in tumour vs normal tissue. In comparison with normal cells at a pHe of 7.4 (a model of cells in normal tissues), acid-adapted cells at a pHe of 6.8 (a model of cells distal from supplying blood vessels in tumours) were more sensitive to chlorambucil, with a dose-modifying factor of approximately 6, and were more resistant to mitoxantrone by a factor of 14.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby B. S. pH studies in human malignant tumours. Lancet. 1966 Aug 6;2(7458):312–315. doi: 10.1016/s0140-6736(66)92598-0. [DOI] [PubMed] [Google Scholar]
- Atema A., Buurman K. J., Noteboom E., Smets L. A. Potentiation of DNA-adduct formation and cytotoxicity of platinum-containing drugs by low pH. Int J Cancer. 1993 Apr 22;54(1):166–172. doi: 10.1002/ijc.2910540126. [DOI] [PubMed] [Google Scholar]
- Brophy G. T., Sladek N. E. Influence of pH on the cytotoxic activity of chlorambucil. Biochem Pharmacol. 1983 Jan 1;32(1):79–84. doi: 10.1016/0006-2952(83)90656-1. [DOI] [PubMed] [Google Scholar]
- Burns C. P., Haugstad B. N., North J. A. Membrane transport of mitoxantrone by L1210 leukemia cells. Biochem Pharmacol. 1987 Mar 15;36(6):857–860. doi: 10.1016/0006-2952(87)90176-6. [DOI] [PubMed] [Google Scholar]
- Chu G. L., Dewey W. C. The role of low intracellular or extracellular pH in sensitization to hyperthermia. Radiat Res. 1988 Apr;114(1):154–167. [PubMed] [Google Scholar]
- Cook J. A., Fox M. H. Effects of chronic pH 6.6 on growth, intracellular pH, and response to 42.0 degrees C hyperthermia of Chinese hamster ovary cells. Cancer Res. 1988 May 1;48(9):2417–2420. [PubMed] [Google Scholar]
- Dennis M. F., Stratford M. R., Wardman P., Watts M. E. Cellular uptake of misonidazole and analogues with acidic or basic functions. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Jun;47(6):629–643. doi: 10.1080/09553008514550871. [DOI] [PubMed] [Google Scholar]
- Gerweck L. E., Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996 Mar 15;56(6):1194–1198. [PubMed] [Google Scholar]
- Helmlinger G., Yuan F., Dellian M., Jain R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997 Feb;3(2):177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- Jähde E., Glüsenkamp K. H., Klünder I., Hülser D. F., Tietze L. F., Rajewsky M. F. Hydrogen ion-mediated enhancement of cytotoxicity of bis-chloroethylating drugs in rat mammary carcinoma cells in vitro. Cancer Res. 1989 Jun 1;49(11):2965–2972. [PubMed] [Google Scholar]
- Jähde E., Glüsenkamp K. H., Rajewsky M. F. Protection of cultured malignant cells from mitoxantrone cytotoxicity by low extracellular pH: a possible mechanism for chemoresistance in vivo. Eur J Cancer. 1990 Feb;26(2):101–106. doi: 10.1016/0277-5379(90)90290-a. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R. B., Asher C., Hicks T. Extracellular pH, transmembrane distribution and cytotoxicity of chlorambucil. Biochem Pharmacol. 1985 Jul 15;34(14):2531–2534. doi: 10.1016/0006-2952(85)90538-6. [DOI] [PubMed] [Google Scholar]
- Parkins C. S., Chadwick J. A., Chaplin D. J. Inhibition of intracellular pH control and relationship to cytotoxicity of chlorambucil and vinblastine. Br J Cancer Suppl. 1996 Jul;27:S75–S77. [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Skarsgard L. D., Chaplin D. J., Wilson D. J., Skwarchuk M. W., Vinczan A., Kristl J. The effect of hypoxia and low pH on the cytotoxicity of chlorambucil. Int J Radiat Oncol Biol Phys. 1992;22(4):737–741. doi: 10.1016/0360-3016(92)90514-i. [DOI] [PubMed] [Google Scholar]
- Skarsgard L. D., Skwarchuk M. W., Vinczan A., Kristl J., Chaplin D. J. The cytotoxicity of melphalan and its relationship to pH, hypoxia and drug uptake. Anticancer Res. 1995 Jan-Feb;15(1):219–223. [PubMed] [Google Scholar]
- Thistlethwaite A. J., Alexander G. A., Moylan D. J., 3rd, Leeper D. B. Modification of human tumor pH by elevation of blood glucose. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):603–610. doi: 10.1016/0360-3016(87)90078-2. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Vukovic V., Tannock I. F. Influence of low pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J Cancer. 1997;75(8):1167–1172. doi: 10.1038/bjc.1997.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M. L., Coss R. A., Bobyock S. B., Leeper D. B., Owen C. S. Thermotolerance and intracellular pH in two Chinese hamster cell lines adapted to growth at low pH. J Cell Physiol. 1996 Feb;166(2):438–445. doi: 10.1002/(SICI)1097-4652(199602)166:2<438::AID-JCP22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Wike-Hooley J. L., Haveman J., Reinhold H. S. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984 Dec;2(4):343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]


