Abstract
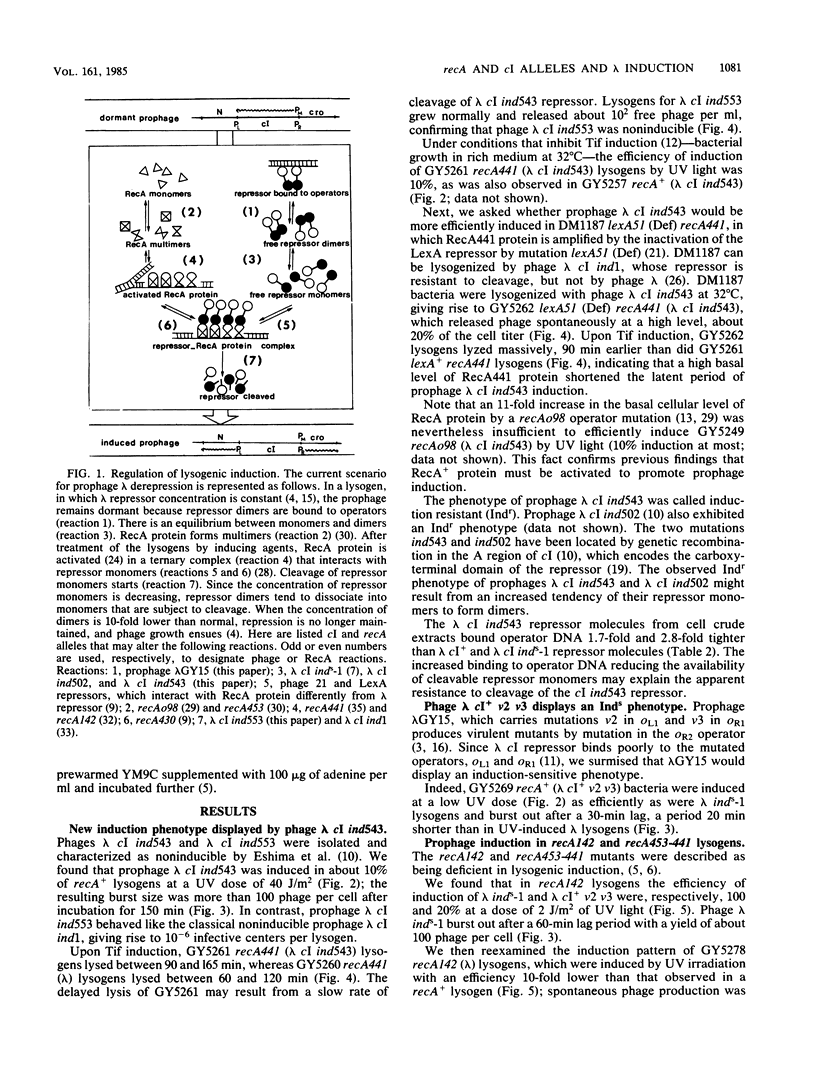
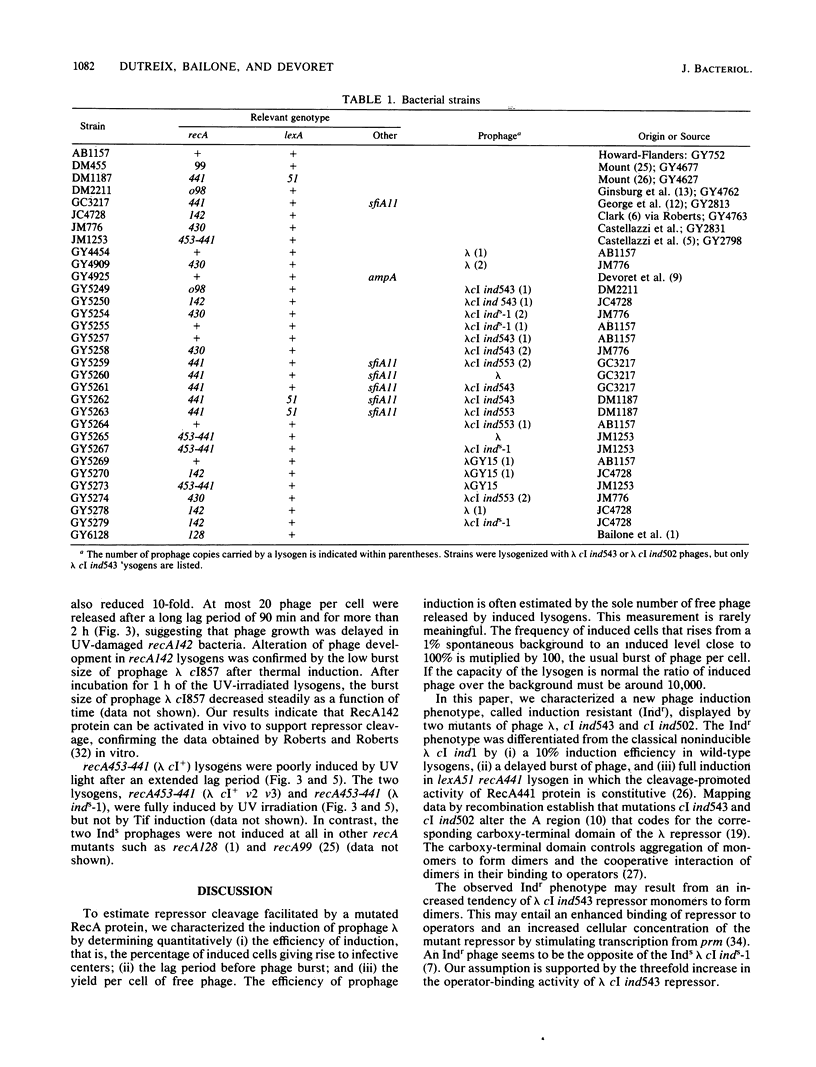
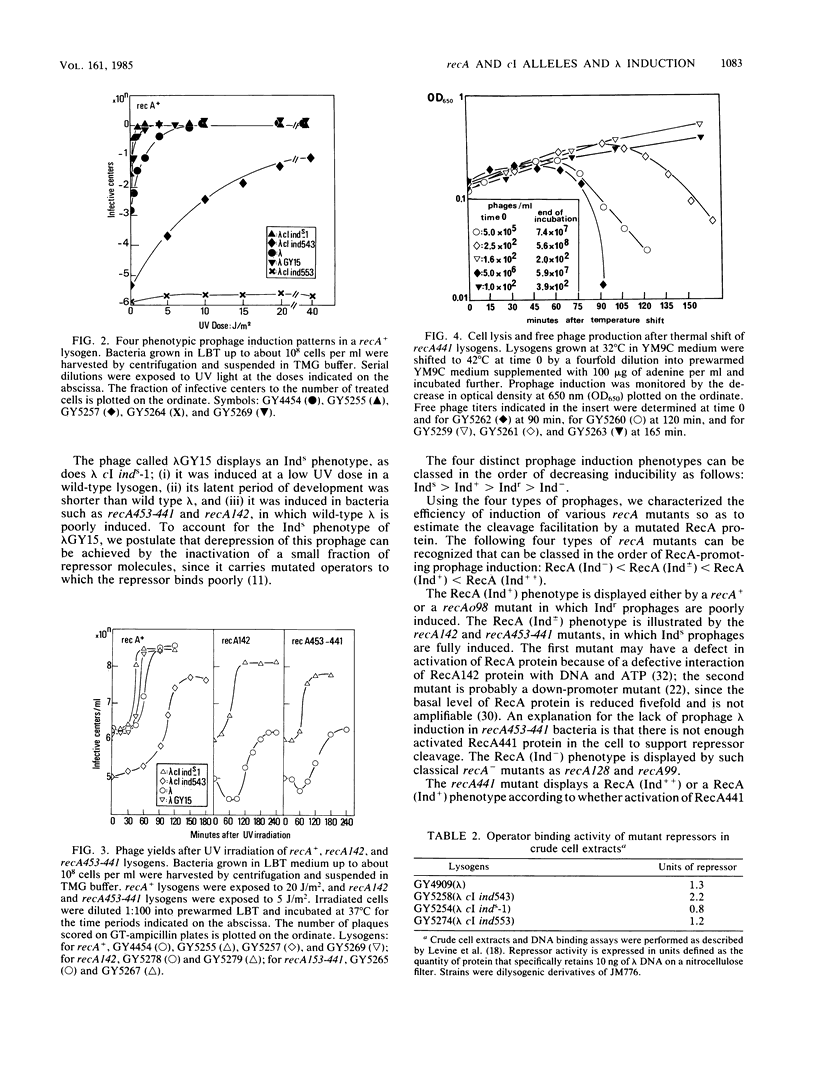
Mutants of the cI gene of prophage lambda have been defined phenotypically in a recA+ host as noninducible (Ind-), inducible (Ind+), or induction sensitive (Inds). We showed that a phage lambda cI+ carrying operator mutations v2 and v3 displays an Inds phenotype, as does lambda cI inds-1. We characterized a fourth induction phenotype called induction resistant (Indr). Using these four prophage types, we tested the influence of bacterial recA mutations on prophage induction. Indr prophages were fully induced in recA441 bacteria whose RecA441 protein is activated constitutively. Indr prophages were not induced in a mutant overproducing RecA+ protein, confirming that RecA+ protein must be activated to promote prophage induction. Inds prophages were induced in recA142 and recA453-441 lysogens, previously described as deficient in prophage induction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailone A., Blanco M., Devoret R. E. coli K12 inf: a mutant deficient in prophage lambda induction and cell filamentation. Mol Gen Genet. 1975;136(4):291–307. doi: 10.1007/BF00341714. [DOI] [PubMed] [Google Scholar]
- Bailone A., Devoret R. Isolation of ultravirulent mutants of phage lambda. Virology. 1978 Feb;84(2):547–550. doi: 10.1016/0042-6822(78)90273-8. [DOI] [PubMed] [Google Scholar]
- Bailone A., Galibert F. Nucleotide sequence of the operators of lambda ultravirulent mutants. Nucleic Acids Res. 1980 May 24;8(10):2147–2164. doi: 10.1093/nar/8.10.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailone A., Levine A., Devoret R. Inactivation of prophage lambda repressor in vivo. J Mol Biol. 1979 Jul 5;131(3):553–572. doi: 10.1016/0022-2836(79)90007-x. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Cohen S., Knoll B. J., Little J. W., Mount D. W. Preferential cleavage of phage lambda repressor monomers by recA protease. Nature. 1981 Nov 12;294(5837):182–184. doi: 10.1038/294182a0. [DOI] [PubMed] [Google Scholar]
- Devoret R., Pierre M., Moreau P. L. Prophage phi 80 is induced in Escherichia coli K12 recA430. Mol Gen Genet. 1983;189(2):199–206. doi: 10.1007/BF00337804. [DOI] [PubMed] [Google Scholar]
- Flashman S. M. Mutational analysis of the operators of bacteriophage lambda. Mol Gen Genet. 1978 Oct 25;166(1):61–73. doi: 10.1007/BF00379730. [DOI] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Ginsburg H., Edmiston S. H., Harper J., Mount D. W. Isolation and characterization of an operator-constitutive mutation in the recA gene of E. coli K-12. Mol Gen Genet. 1982;187(1):4–11. doi: 10.1007/BF00384376. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Inokuchi H. Temperature-sensitive regulation system of prophage lambda induction. J Mol Biol. 1967 Jan 28;23(2):217–224. doi: 10.1016/s0022-2836(67)80029-9. [DOI] [PubMed] [Google Scholar]
- Levine A., Bailone A., Devoret R. Cellular levels of the prophage lambda and 434 repressors. J Mol Biol. 1979 Jul 5;131(3):655–661. doi: 10.1016/0022-2836(79)90014-7. [DOI] [PubMed] [Google Scholar]
- Lieb M. A fine structure map of spontaneous and induced mutations in the lambda repressor gene, including insertions of IS elements. Mol Gen Genet. 1981;184(3):364–371. doi: 10.1007/BF00352506. [DOI] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Moreau P. L., Fanica M., Devoret R. Induction of prophage lambda does not require full induction of RecA protein synthesis. Biochimie. 1980;62(10):687–694. doi: 10.1016/s0300-9084(80)80026-5. [DOI] [PubMed] [Google Scholar]
- Moreau P., Bailone A., Devoret R. Prophage lambda induction of Escherichia coli K12 envA uvrB: a highly sensitive test for potential carcinogens. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3700–3704. doi: 10.1073/pnas.73.10.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. Isolation and genetic analysis of a strain of Escherichia coli K-12 with an amber recA mutation. J Bacteriol. 1971 Jul;107(1):388–389. doi: 10.1128/jb.107.1.388-389.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T., Sturtevant J. M., Ptashne M. The lambda repressor contains two domains. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1608–1612. doi: 10.1073/pnas.76.4.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky E. M., Roberts J. W. Kinetics of RecA protein-directed inactivation of repressors of phage lambda and phage P22. J Mol Biol. 1980 May 25;139(3):319–328. doi: 10.1016/0022-2836(80)90133-3. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Moreau P. L., Ginsburg H., Mount D. W., Devoret R. Cell survival, UV-reactivation and induction of prophage lambda in Escherichia coli K12 overproducing RecA protein. Mol Gen Genet. 1982;188(1):37–43. doi: 10.1007/BF00332993. [DOI] [PubMed] [Google Scholar]
- Rebollo J. E., Moreau P. L., Blanco M., Devoret R. Restoration of RecA protein activity by genetic complementation. Mol Gen Genet. 1984;195(1-2):83–89. doi: 10.1007/BF00332728. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Two mutations that alter the regulatory activity of E. coli recA protein. Nature. 1981 Apr 2;290(5805):422–424. doi: 10.1038/290422a0. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Pabo C. O., Meyer B. J., Ptashne M., Backman K. C. Regulatory functions of the lambda repressor reside in the amino-terminal domain. Nature. 1979 May 31;279(5712):396–400. doi: 10.1038/279396a0. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M. Enzymatic activities of the RecA protein of Escherichia coli. Biochimie. 1982 Aug-Sep;64(8-9):611–616. doi: 10.1016/s0300-9084(82)80098-9. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. From Gainesville to Toulouse: the evolution of a model. Biochimie. 1982 Aug-Sep;64(8-9):549–555. doi: 10.1016/s0300-9084(82)80086-2. [DOI] [PubMed] [Google Scholar]



