Abstract
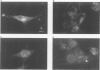
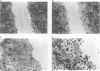
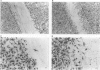
The expression of hepatocyte growth factor/scatter factor (HGF/SF) was studied in 12 mesothelioma cell lines characterized by either an epithelioid or a fibroblast-like phenotype. Conditioned media from these lines were analysed by bioassay and ELISA, and HGF/SF was detected in three cell lines, all with a fibroblast-like or mixed morphology. None of eight epithelioid cell lines expressed the factor. Thus, for these cell lines, the ability to secrete HGF/SF correlated with the cell phenotype. Following on from these observations, two cell lines, BR and BT, with a fibroblast-like and an epithelioid phenotype, respectively, were further investigated. Both cell lines expressed the Met receptor but only BR secreted HGF/SF. Both cell lines responded to exogenous HGF/SF treatment by a change of morphology but in different ways: BR became more elongated and bipolar, while BT formed more spread-out cell colonies. HGF/SF acted as a paracrine effector on the epithelioid BT cells and stimulated both cell-spreading and proliferation. Interestingly, BT cells spread but did not scatter in response to exogenous HGF/SF. In contrast BR cells showed only some stimulation of cell motility with HGF/SF and no increase in cell proliferation was observed. Because HGF/SF was previously found in the pleural effusion fluids of patients with malignant mesothelioma and in paraffin-embedded tumour tissues, it is concluded that HGF/SF may well stimulate the growth and spread of malignant mesothelioma in vivo by paracrine and/or autocrine mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Fernandez E., Diez-Nau M. D. Malignant fibrosarcomatous mesothelioma and benign pleural fibroma (localized fibrous mesothelioma) in tissue culture: a comparison of the in vitro pattern of growth in relation to the cell of origin. Cancer. 1979 May;43(5):1658–1663. doi: 10.1002/1097-0142(197905)43:5<1658::aid-cncr2820430515>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Andermarcher E., Surani M. A., Gherardi E. Co-expression of the HGF/SF and c-met genes during early mouse embryogenesis precedes reciprocal expression in adjacent tissues during organogenesis. Dev Genet. 1996;18(3):254–266. doi: 10.1002/(SICI)1520-6408(1996)18:3<254::AID-DVG6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Arakaki N., Kawakami S., Nakamura O., Ohnishi T., Miyazaki H., Ishii T., Tsubouchi H., Daikuhara Y. Evidence for the presence of an inactive precursor of human hepatocyte growth factor in plasma and sera of patients with liver diseases. Hepatology. 1995 Dec;22(6):1728–1734. [PubMed] [Google Scholar]
- Asplund T., Versnel M. A., Laurent T. C., Heldin P. Human mesothelioma cells produce factors that stimulate the production of hyaluronan by mesothelial cells and fibroblasts. Cancer Res. 1993 Jan 15;53(2):388–392. [PubMed] [Google Scholar]
- Bielefeldt-Ohmann H., Jarnicki A. G., Fitzpatrick D. R. Molecular pathobiology and immunology of malignant mesothelioma. J Pathol. 1996 Apr;178(4):369–378. doi: 10.1002/(SICI)1096-9896(199604)178:4<369::AID-PATH460>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Current pathogenetic concepts of diffuse malignant mesothelioma. Hum Pathol. 1987 Jun;18(6):544–557. doi: 10.1016/s0046-8177(87)80354-4. [DOI] [PubMed] [Google Scholar]
- Davis J. M. The histopathology and ultrastructure of pleural mesotheliomas produced in the rat by injections of crocidolite asbestos. Br J Exp Pathol. 1979 Dec;60(6):642–652. [PMC free article] [PubMed] [Google Scholar]
- Di Renzo M. F., Narsimhan R. P., Olivero M., Bretti S., Giordano S., Medico E., Gaglia P., Zara P., Comoglio P. M. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991 Nov;6(11):1997–2003. [PubMed] [Google Scholar]
- Di Renzo M. F., Poulsom R., Olivero M., Comoglio P. M., Lemoine N. R. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995 Mar 1;55(5):1129–1138. [PubMed] [Google Scholar]
- Dowrick P. G., Prescott A. R., Warn R. M. Scatter factor affects major changes in the cytoskeletal organization of epithelial cells. Cytokine. 1991 Jul;3(4):299–310. doi: 10.1016/1043-4666(91)90498-3. [DOI] [PubMed] [Google Scholar]
- Duncan G., Riach R. A., Williams M. R., Webb S. F., Dawson A. P., Reddan J. R. Calcium mobilisation modulates growth of lens cells. Cell Calcium. 1996 Jan;19(1):83–89. doi: 10.1016/s0143-4160(96)90015-9. [DOI] [PubMed] [Google Scholar]
- Eagles G., Warn A., Ball R. Y., Baillie-Johnson H., Arakaki N., Daikuhara Y., Warn R. M. Hepatocyte growth factor/scatter factor is present in most pleural effusion fluids from cancer patients. Br J Cancer. 1996 Feb;73(3):377–381. doi: 10.1038/bjc.1996.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Peroni D. J., Bielefeldt-Ohmann H. The role of growth factors and cytokines in the tumorigenesis and immunobiology of malignant mesothelioma. Am J Respir Cell Mol Biol. 1995 May;12(5):455–460. doi: 10.1165/ajrcmb.12.5.7742009. [DOI] [PubMed] [Google Scholar]
- Fleury-Feith J., Kheuang L., Zeng L., Bignon J., Boutin C., Monnet I., Jaurand M. C. Human malignant mesothelial cells: variability of ultrastructural features in established and nude mice transplanted cell lines. J Pathol. 1995 Oct;177(2):209–215. doi: 10.1002/path.1711770215. [DOI] [PubMed] [Google Scholar]
- Gak E., Taylor W. G., Chan A. M., Rubin J. S. Processing of hepatocyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett. 1992 Oct 12;311(1):17–21. doi: 10.1016/0014-5793(92)81356-q. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Lechner J. F., Reddel R. R., Roberts A. B., Robbins K. C., Gabrielson E. W., Harris C. C. Comparison of production of transforming growth factor-beta and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res. 1987 Dec 1;47(23):6180–6184. [PubMed] [Google Scholar]
- Gherardi E., Sharpe M., Lane K., Sirulnik A., Stoker M. Hepatocyte growth factor/scatter factor (HGF/SF), the c-met receptor and the behaviour of epithelial cells. Symp Soc Exp Biol. 1993;47:163–181. [PubMed] [Google Scholar]
- Han S., Stuart L. A., Degen S. J. Characterization of the DNF15S2 locus on human chromosome 3: identification of a gene coding for four kringle domains with homology to hepatocyte growth factor. Biochemistry. 1991 Oct 8;30(40):9768–9780. doi: 10.1021/bi00104a029. [DOI] [PubMed] [Google Scholar]
- Harvey P., Warn A., Newman P., Perry L. J., Ball R. Y., Warn R. M. Immunoreactivity for hepatocyte growth factor/scatter factor and its receptor, met, in human lung carcinomas and malignant mesotheliomas. J Pathol. 1996 Dec;180(4):389–394. doi: 10.1002/(SICI)1096-9896(199612)180:4<389::AID-PATH685>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Cawston T. E., Dingle J. T., Reynolds J. J. Characterization of a specific antiserum for mammalian collagenase from several species: immunolocalization of collagenase in rabbit chondrocytes and uterus. J Cell Sci. 1986 Mar;81:105–123. doi: 10.1242/jcs.81.1.105. [DOI] [PubMed] [Google Scholar]
- Hillerdal G. Malignant mesothelioma 1982: review of 4710 published cases. Br J Dis Chest. 1983 Oct;77(4):321–343. [PubMed] [Google Scholar]
- Joseph A., Weiss G. H., Jin L., Fuchs A., Chowdhury S., O'Shaugnessy P., Goldberg I. D., Rosen E. M. Expression of scatter factor in human bladder carcinoma. J Natl Cancer Inst. 1995 Mar 1;87(5):372–377. doi: 10.1093/jnci/87.5.372. [DOI] [PubMed] [Google Scholar]
- Kolatsi-Joannou M., Woolf A. S., Hardman P., White S. J., Gordge M., Henderson R. M. The hepatocyte growth factor/scatter factor (HGF/SF) receptor, met, transduces a morphogenetic signal in renal glomerular fibromuscular mesangial cells. J Cell Sci. 1995 Dec;108(Pt 12):3703–3714. doi: 10.1242/jcs.108.12.3703. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Zhang Y., Aston C., Hintz R., Jagirdar J., Perle M. A., Burt M., Rom W. N. Normal human mesothelial cells and mesothelioma cell lines express insulin-like growth factor I and associated molecules. Cancer Res. 1993 Jun 15;53(12):2858–2864. [PubMed] [Google Scholar]
- Miyazawa K., Shimomura T., Kitamura A., Kondo J., Morimoto Y., Kitamura N. Molecular cloning and sequence analysis of the cDNA for a human serine protease reponsible for activation of hepatocyte growth factor. Structural similarity of the protease precursor to blood coagulation factor XII. J Biol Chem. 1993 May 15;268(14):10024–10028. [PubMed] [Google Scholar]
- Miyazawa K., Tsubouchi H., Naka D., Takahashi K., Okigaki M., Arakaki N., Nakayama H., Hirono S., Sakiyama O., Takahashi K. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989 Sep 15;163(2):967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989 Nov 23;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Naldini L., Tamagnone L., Vigna E., Sachs M., Hartmann G., Birchmeier W., Daikuhara Y., Tsubouchi H., Blasi F., Comoglio P. M. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992 Dec;11(13):4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Di Renzo M. F., Prat M., Bigotti A., Cavaliere R., Comoglio P. M. Expression of the c-Met/HGF receptor in human melanocytic neoplasms: demonstration of the relationship to malignant melanoma tumour progression. Br J Cancer. 1993 Oct;68(4):746–750. doi: 10.1038/bjc.1993.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto J., Hodgson J. T., Matthews F. E., Jones J. R. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995 Mar 4;345(8949):535–539. doi: 10.1016/s0140-6736(95)90462-x. [DOI] [PubMed] [Google Scholar]
- Prat M., Narsimhan R. P., Crepaldi T., Nicotra M. R., Natali P. G., Comoglio P. M. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991 Sep 30;49(3):323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- Rong S., Bodescot M., Blair D., Dunn J., Nakamura T., Mizuno K., Park M., Chan A., Aaronson S., Vande Woude G. F. Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol Cell Biol. 1992 Nov;12(11):5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong S., Jeffers M., Resau J. H., Tsarfaty I., Oskarsson M., Vande Woude G. F. Met expression and sarcoma tumorigenicity. Cancer Res. 1993 Nov 15;53(22):5355–5360. [PubMed] [Google Scholar]
- Rosen E. M., Nigam S. K., Goldberg I. D. Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol. 1994 Dec;127(6 Pt 2):1783–1787. doi: 10.1083/jcb.127.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg E., Meyer D., Weidner K. M., Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993 Oct;123(1):223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985 Aug;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- Tsarfaty I., Rong S., Resau J. H., Rulong S., da Silva P. P., Vande Woude G. F. The Met proto-oncogene mesenchymal to epithelial cell conversion. Science. 1994 Jan 7;263(5143):98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- Tsubouchi H., Niitani Y., Hirono S., Nakayama H., Gohda E., Arakaki N., Sakiyama O., Takahashi K., Kimoto M., Kawakami S. Levels of the human hepatocyte growth factor in serum of patients with various liver diseases determined by an enzyme-linked immunosorbent assay. Hepatology. 1991 Jan;13(1):1–5. [PubMed] [Google Scholar]
- Versnel M. A., Hagemeijer A., Bouts M. J., van der Kwast T. H., Hoogsteden H. C. Expression of c-sis (PDGF B-chain) and PDGF A-chain genes in ten human malignant mesothelioma cell lines derived from primary and metastatic tumors. Oncogene. 1988 Jun;2(6):601–605. [PubMed] [Google Scholar]
- Webb C. P., Lane K., Dawson A. P., Vande Woude G. F., Warn R. M. C-Met signalling in an HGF/SF-insensitive variant MDCK cell line with constitutive motile/invasive behaviour. J Cell Sci. 1996 Sep;109(Pt 9):2371–2381. doi: 10.1242/jcs.109.9.2371. [DOI] [PubMed] [Google Scholar]
- Whitaker D., Papadimitriou J. M., Walters M. N. The mesothelium and its reactions: a review. Crit Rev Toxicol. 1982 Apr;10(2):81–144. doi: 10.3109/10408448209041321. [DOI] [PubMed] [Google Scholar]
- Woolf A. S., Kolatsi-Joannou M., Hardman P., Andermarcher E., Moorby C., Fine L. G., Jat P. S., Noble M. D., Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995 Jan;128(1-2):171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J., Ogawa M., Yamashita S., Nomura K., Kuramoto M., Saishoji T., Shin S. Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994 Apr 1;54(7):1630–1633. [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Wang M. H., Skeel A., Leonard E. J. Cloning, sequencing, and expression of human macrophage stimulating protein (MSP, MST1) confirms MSP as a member of the family of kringle proteins and locates the MSP gene on chromosome 3. J Biol Chem. 1993 Jul 25;268(21):15461–15468. [PubMed] [Google Scholar]
- Zeng L., Fleury-Feith J., Monnet I., Boutin C., Bignon J., Jaurand M. C. Immunocytochemical characterization of cell lines from human malignant mesothelioma: characterization of human mesothelioma cell lines by immunocytochemistry with a panel of monoclonal antibodies. Hum Pathol. 1994 Mar;25(3):227–234. doi: 10.1016/0046-8177(94)90192-9. [DOI] [PubMed] [Google Scholar]
- van Gelder T., Hoogsteden H. C., Vandenbroucke J. P., van der Kwast T. H., Planteydt H. T. The influence of the diagnostic technique on the histopathological diagnosis in malignant mesothelioma. Virchows Arch A Pathol Anat Histopathol. 1991;418(4):315–317. doi: 10.1007/BF01600160. [DOI] [PubMed] [Google Scholar]