Abstract
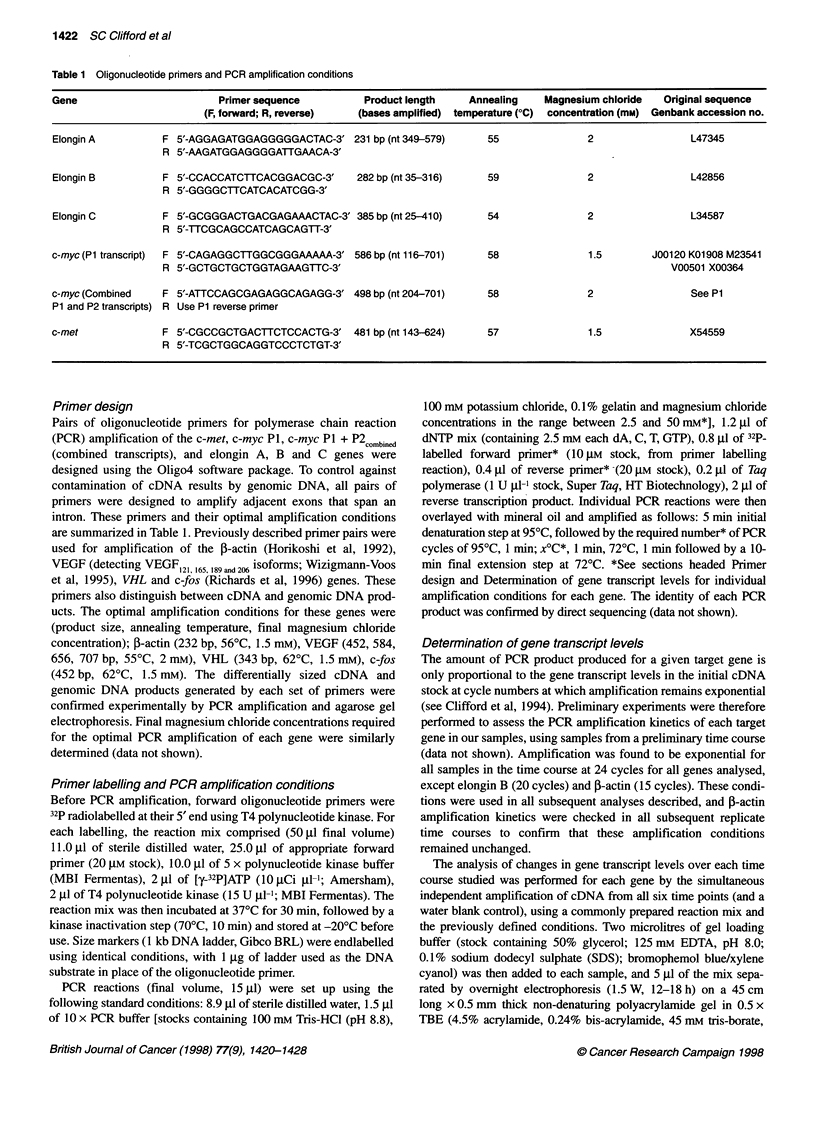
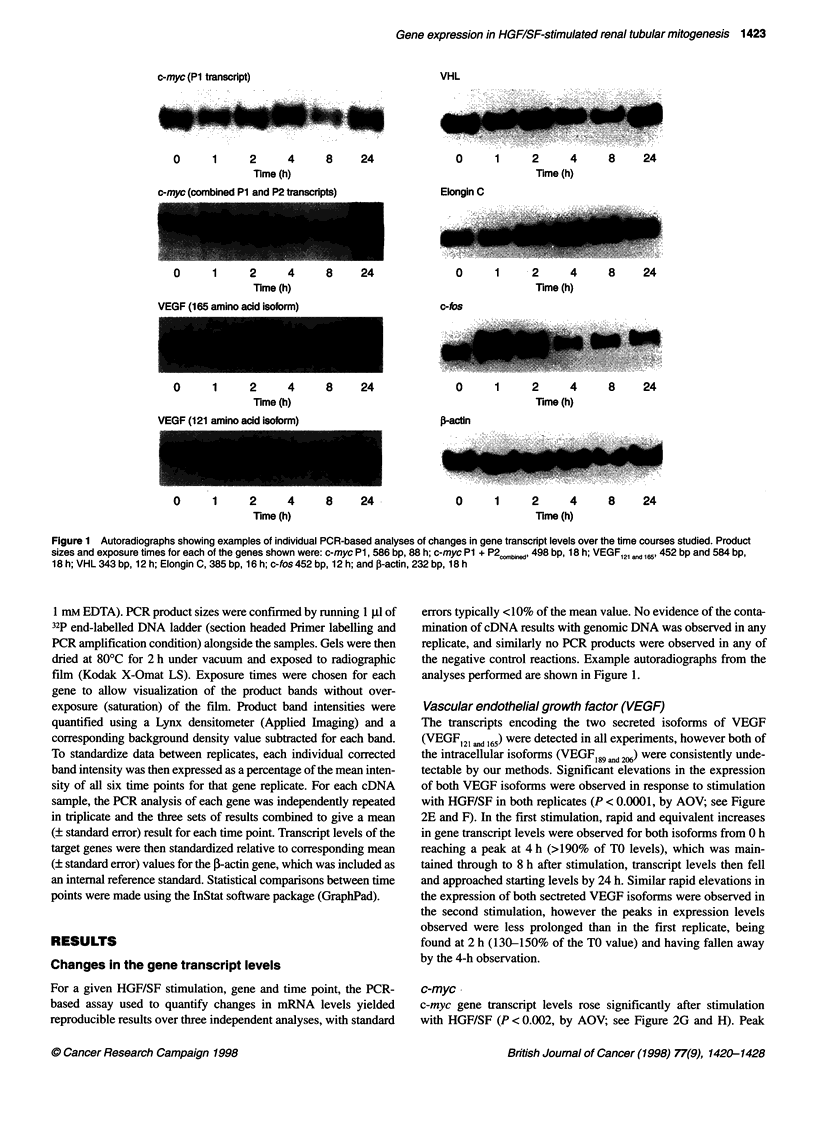
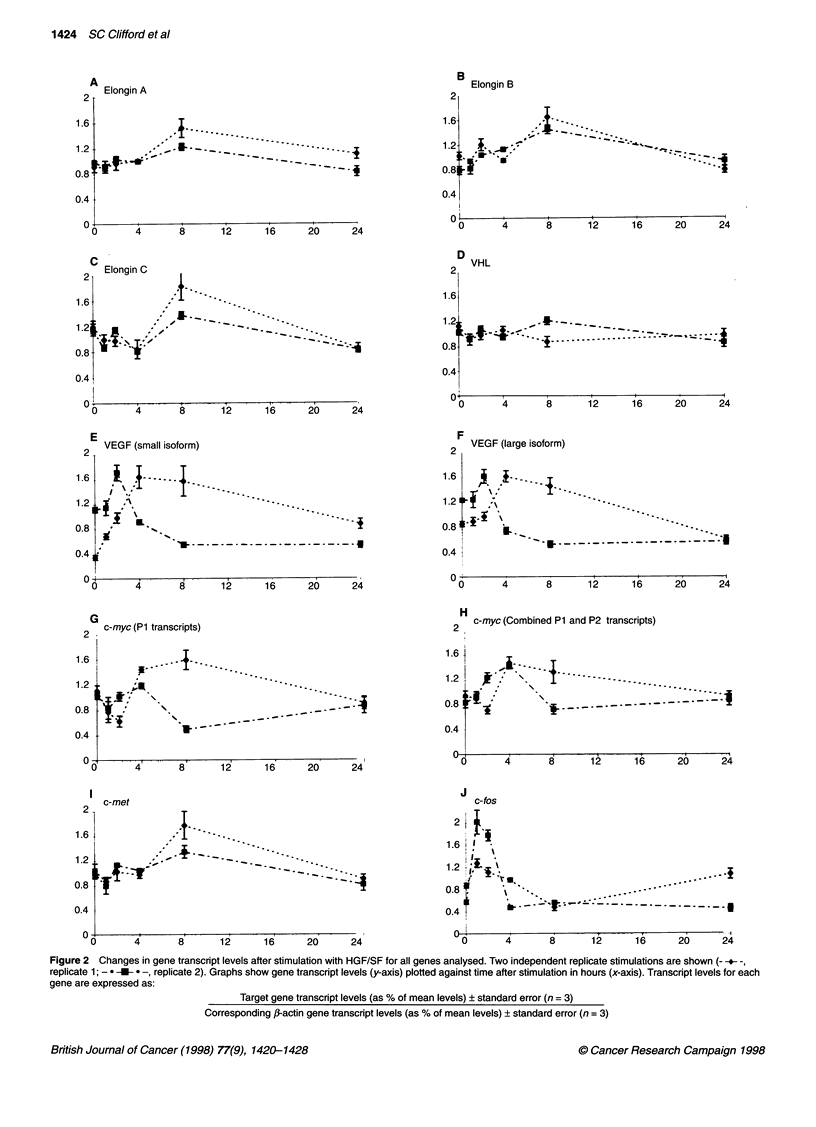
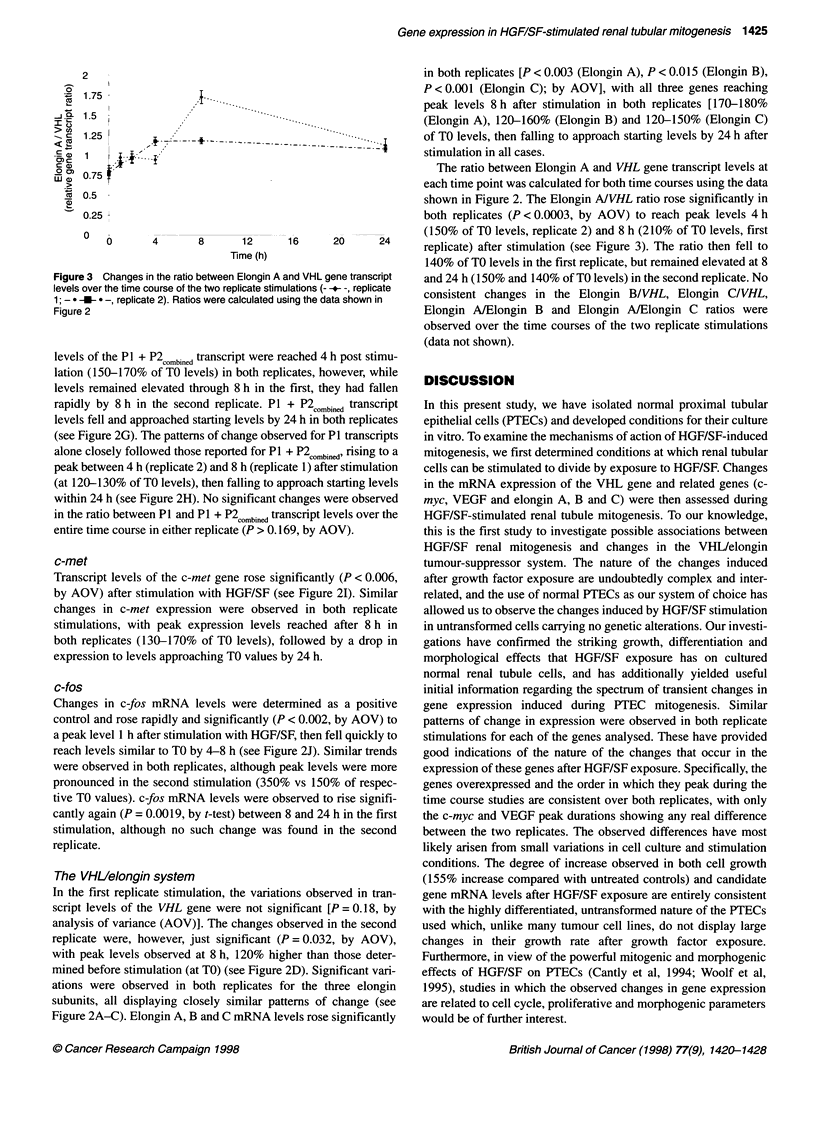
Hepatocyte growth factor (HGF/SF) is a potent renal proximal tubular cell (PTEC) mitogen involved in renal development. HGF/SF is the functional ligand for the c-met proto-oncogene, and germline c-met mutations are associated with familial papillary renal cell carcinoma. Somatic von Hippel-Lindau disease tumour-suppressor gene (VHL) mutations are frequently detected in sporadic clear cell renal cell carcinomas (RCC), and germline VHL mutations are the commonest cause of familial clear cell RCC. pVHL binds to the positive regulatory components of the trimeric elongin (SIII) complex (elongins B and C) and has been observed to deregulate expression of the vascular endothelial growth factor (VEGF) gene. HGF/SF has similarly been reported to up-regulate expression of the VEGF gene in non-renal experimental systems. To investigate the mechanism of HGF/SF action in PTECs and, specifically, to examine potential interactions between the HGF/c-met and the VHL-mediated pathways for renal tubular growth control, we have isolated untransformed PTECs from normal kidneys, developed conditions for their culture in vitro and used these cells to investigate changes in mRNA levels of the VHL, elongin A, B and C, VEGF, c-myc, c-fos and c-met genes after HGF/SF exposure. Significant elevations in the mRNA levels of VEGF, c-myc, c-fos, c-met and elongins A, B and C, but not VHL, were detected after HGF/SF stimulation of human PTECs (P < 0.02), with a consistent order of peak levels observed over successive replicates (c-fos at 1 h, VEGF at 2-4 h, c-myc, at 4 h, followed by c-met and all three elongin subunits at 8 h). This study highlights the spectrum of changes in gene expression observed in PTECs after HGF/SF stimulation and has identified possible candidate mediators of the HGF/SF-induced mitogenic response. Our evidence would suggest that the changes in PTEC VEGF expression induced by HGF/SF are mediated by a VHL-independent pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aso T., Lane W. S., Conaway J. W., Conaway R. C. Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II. Science. 1995 Sep 8;269(5229):1439–1443. doi: 10.1126/science.7660129. [DOI] [PubMed] [Google Scholar]
- Boccaccio C., Gaudino G., Gambarotta G., Galimi F., Comoglio P. M. Hepatocyte growth factor (HGF) receptor expression is inducible and is part of the delayed-early response to HGF. J Biol Chem. 1994 Apr 29;269(17):12846–12851. [PubMed] [Google Scholar]
- Breier G., Albrecht U., Sterrer S., Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992 Feb;114(2):521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Dvorak H. F., Senger D. R. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993 Nov;143(5):1255–1262. [PMC free article] [PubMed] [Google Scholar]
- Cantley L. G., Barros E. J., Gandhi M., Rauchman M., Nigam S. K. Regulation of mitogenesis, motogenesis, and tubulogenesis by hepatocyte growth factor in renal collecting duct cells. Am J Physiol. 1994 Aug;267(2 Pt 2):F271–F280. doi: 10.1152/ajprenal.1994.267.2.F271. [DOI] [PubMed] [Google Scholar]
- Chen F., Kishida T., Duh F. M., Renbaum P., Orcutt M. L., Schmidt L., Zbar B. Suppression of growth of renal carcinoma cells by the von Hippel-Lindau tumor suppressor gene. Cancer Res. 1995 Nov 1;55(21):4804–4807. [PubMed] [Google Scholar]
- Clifford S. C., Thomas D. J., Neal D. E., Lunec J. Increased mdr1 gene transcript levels in high-grade carcinoma of the bladder determined by quantitative PCR-based assay. Br J Cancer. 1994 Apr;69(4):680–686. doi: 10.1038/bjc.1994.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D. T., Heuvelman D. M., Nelson R., Olander J. V., Eppley B. L., Delfino J. J., Siegel N. R., Leimgruber R. M., Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989 Nov;84(5):1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrisac C. J., Sens M. A., Garvin A. J., Spicer S. S., Sens D. A. Tissue culture of human kidney epithelial cells of proximal tubule origin. Kidney Int. 1984 Feb;25(2):383–390. doi: 10.1038/ki.1984.28. [DOI] [PubMed] [Google Scholar]
- Dressler G. R., Douglass E. C. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D. R., Pause A., Burgess W. H., Aso T., Chen D. Y., Garrett K. P., Conaway R. C., Conaway J. W., Linehan W. M., Klausner R. D. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995 Sep 8;269(5229):1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- Eick D., Polack A., Kofler E., Lenoir G. M., Rickinson A. B., Bornkamm G. W. Expression of P0- and P3-RNA from the normal and translocated c-myc allele in Burkitt's lymphoma cells. Oncogene. 1990 Sep;5(9):1397–1402. [PubMed] [Google Scholar]
- Ferrara N., Houck K. A., Jakeman L. B., Winer J., Leung D. W. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991 Nov;47(3):211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- Finkenzeller G., Technau A., Marmé D. Hypoxia-induced transcription of the vascular endothelial growth factor gene is independent of functional AP-1 transcription factor. Biochem Biophys Res Commun. 1995 Mar 8;208(1):432–439. doi: 10.1006/bbrc.1995.1356. [DOI] [PubMed] [Google Scholar]
- Foster K., Prowse A., van den Berg A., Fleming S., Hulsbeek M. M., Crossey P. A., Richards F. M., Cairns P., Affara N. A., Ferguson-Smith M. A. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994 Dec;3(12):2169–2173. doi: 10.1093/hmg/3.12.2169. [DOI] [PubMed] [Google Scholar]
- Gnarra J. R., Dressler G. R. Expression of Pax-2 in human renal cell carcinoma and growth inhibition by antisense oligonucleotides. Cancer Res. 1995 Sep 15;55(18):4092–4098. [PubMed] [Google Scholar]
- Gnarra J. R., Tory K., Weng Y., Schmidt L., Wei M. H., Li H., Latif F., Liu S., Chen F., Duh F. M. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994 May;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- Gnarra J. R., Zhou S., Merrill M. J., Wagner J. R., Krumm A., Papavassiliou E., Oldfield E. H., Klausner R. D., Linehan W. M. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):10589–10594. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grugel S., Finkenzeller G., Weindel K., Barleon B., Marmé D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995 Oct 27;270(43):25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Burns K. D., Alattar M., Homma T., Nakamura T. Hepatocyte growth factor stimulates phosphoinositide hydrolysis and mitogenesis in cultured renal epithelial cells. Life Sci. 1993;52(13):1091–1100. doi: 10.1016/0024-3205(93)90430-b. [DOI] [PubMed] [Google Scholar]
- Herman J. G., Latif F., Weng Y., Lerman M. I., Zbar B., Liu S., Samid D., Duan D. S., Gnarra J. R., Linehan W. M. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi T., Danenberg K. D., Stadlbauer T. H., Volkenandt M., Shea L. C., Aigner K., Gustavsson B., Leichman L., Frösing R., Ray M. Quantitation of thymidylate synthase, dihydrofolate reductase, and DT-diaphorase gene expression in human tumors using the polymerase chain reaction. Cancer Res. 1992 Jan 1;52(1):108–116. [PubMed] [Google Scholar]
- Ikeda E., Achen M. G., Breier G., Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995 Aug 25;270(34):19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O., Kibel A., Gray S., Kaelin W. G., Jr Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995 Aug;1(8):822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O., Levy A. P., Jiang C., Kaelin W. G., Jr, Goldberg M. A. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M., Kochhar K., Nakamura T., Iyer A. Hepatocyte growth factor-induced signal transduction in two normal mouse epithelial cell lines. Biochem Mol Biol Int. 1995 Jul;36(3):465–474. [PubMed] [Google Scholar]
- Kawaida K., Matsumoto K., Shimazu H., Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kibel A., Iliopoulos O., DeCaprio J. A., Kaelin W. G., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995 Sep 8;269(5229):1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- Kohlhuber F., Strobl L. J., Eick D. Early down-regulation of c-myc in dimethylsulfoxide-induced mouse erythroleukemia (MEL) cells is mediated at the P1/P2 promoters. Oncogene. 1993 Apr;8(4):1099–1102. [PubMed] [Google Scholar]
- Krumm A., Meulia T., Brunvand M., Groudine M. The block to transcriptional elongation within the human c-myc gene is determined in the promoter-proximal region. Genes Dev. 1992 Nov;6(11):2201–2213. doi: 10.1101/gad.6.11.2201. [DOI] [PubMed] [Google Scholar]
- Kuzmin I., Duh F. M., Latif F., Geil L., Zbar B., Lerman M. I. Identification of the promoter of the human von Hippel-Lindau disease tumor suppressor gene. Oncogene. 1995 Jun 1;10(11):2185–2194. [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Lee S., Chen D. Y., Humphrey J. S., Gnarra J. R., Linehan W. M., Klausner R. D. Nuclear/cytoplasmic localization of the von Hippel-Lindau tumor suppressor gene product is determined by cell density. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1770–1775. doi: 10.1073/pnas.93.5.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Maher E. R., Yates J. R., Harries R., Benjamin C., Harris R., Moore A. T., Ferguson-Smith M. A. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990 Nov;77(283):1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- Pause A., Lee S., Worrell R. A., Chen D. Y., Burgess W. H., Linehan W. M., Klausner R. D. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak J., Mitsuhashi Y., Bayko L., Filmus J., Shirasawa S., Sasazuki T., Kerbel R. S. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995 Oct 15;55(20):4575–4580. [PubMed] [Google Scholar]
- Richards F. M., Schofield P. N., Fleming S., Maher E. R. Expression of the von Hippel-Lindau disease tumour suppressor gene during human embryogenesis. Hum Mol Genet. 1996 May;5(5):639–644. doi: 10.1093/hmg/5.5.639. [DOI] [PubMed] [Google Scholar]
- Schmidt L., Duh F. M., Chen F., Kishida T., Glenn G., Choyke P., Scherer S. W., Zhuang Z., Lubensky I., Dean M. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997 May;16(1):68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- Shuin T., Kondo K., Torigoe S., Kishida T., Kubota Y., Hosaka M., Nagashima Y., Kitamura H., Latif F., Zbar B. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994 Jun 1;54(11):2852–2855. [PubMed] [Google Scholar]
- Siemeister G., Weindel K., Mohrs K., Barleon B., Martiny-Baron G., Marmé D. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 1996 May 15;56(10):2299–2301. [PubMed] [Google Scholar]
- Silvagno F., Follenzi A., Arese M., Prat M., Giraudo E., Gaudino G., Camussi G., Comoglio P. M., Bussolino F. In vivo activation of met tyrosine kinase by heterodimeric hepatocyte growth factor molecule promotes angiogenesis. Arterioscler Thromb Vasc Biol. 1995 Nov;15(11):1857–1865. doi: 10.1161/01.atv.15.11.1857. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Cancer Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Wizigmann-Voos S., Breier G., Risau W., Plate K. H. Up-regulation of vascular endothelial growth factor and its receptors in von Hippel-Lindau disease-associated and sporadic hemangioblastomas. Cancer Res. 1995 Mar 15;55(6):1358–1364. [PubMed] [Google Scholar]
- Woolf A. S., Kolatsi-Joannou M., Hardman P., Andermarcher E., Moorby C., Fine L. G., Jat P. S., Noble M. D., Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995 Jan;128(1-2):171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Shuin T., Misaki H., Kubota Y. Enhanced expression of c-myc and epidermal growth factor receptor (C-erbB-1) genes in primary human renal cancer. Cancer Res. 1988 Dec 1;48(23):6753–6757. [PubMed] [Google Scholar]