Abstract
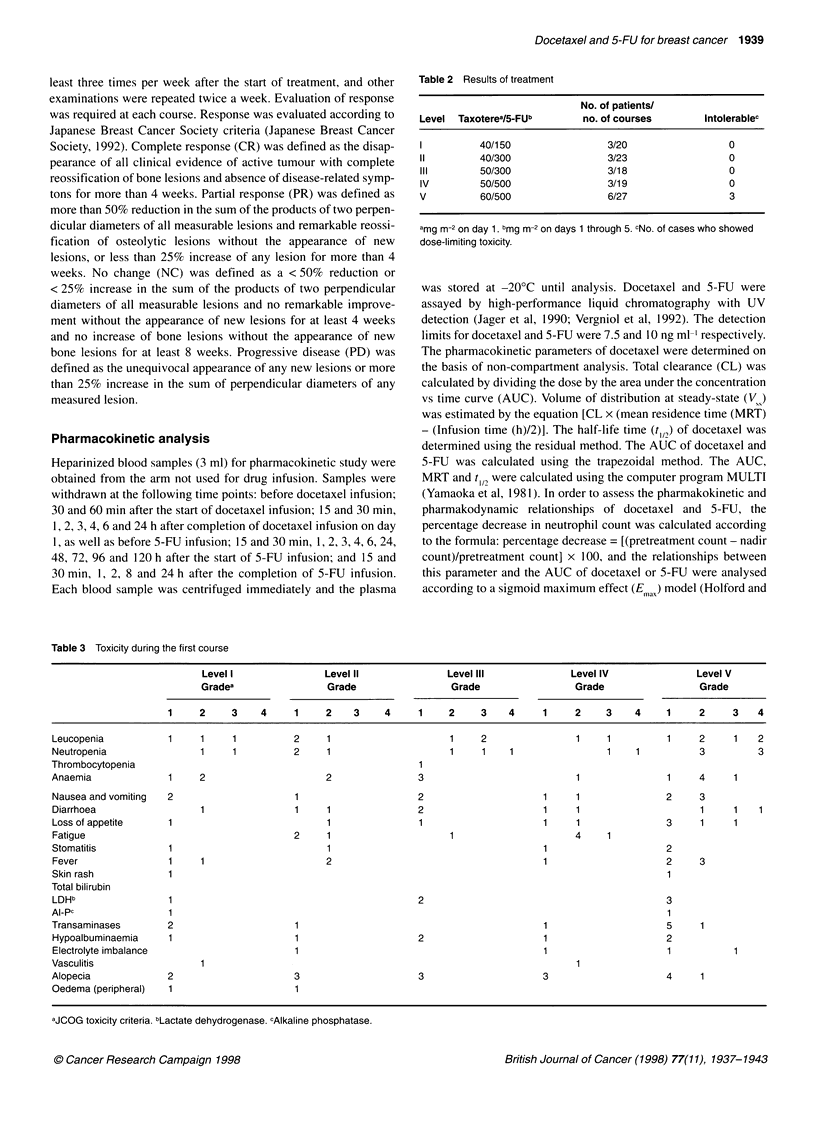
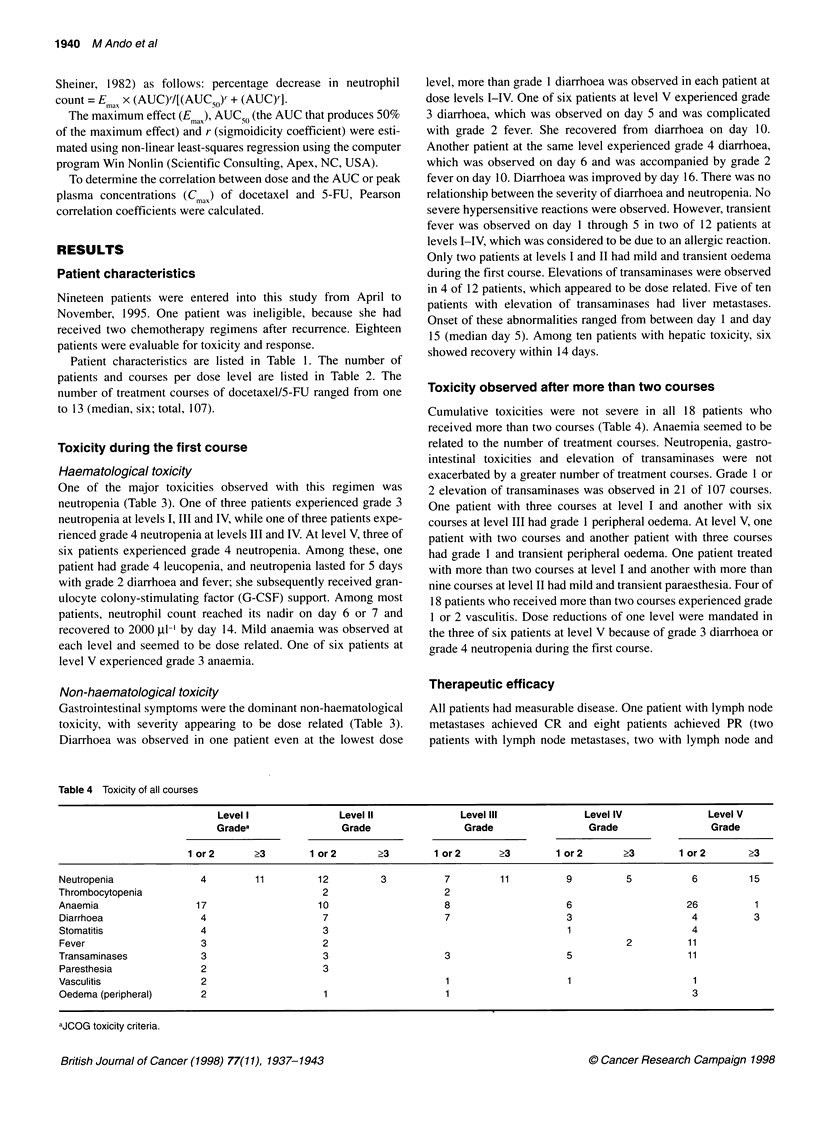
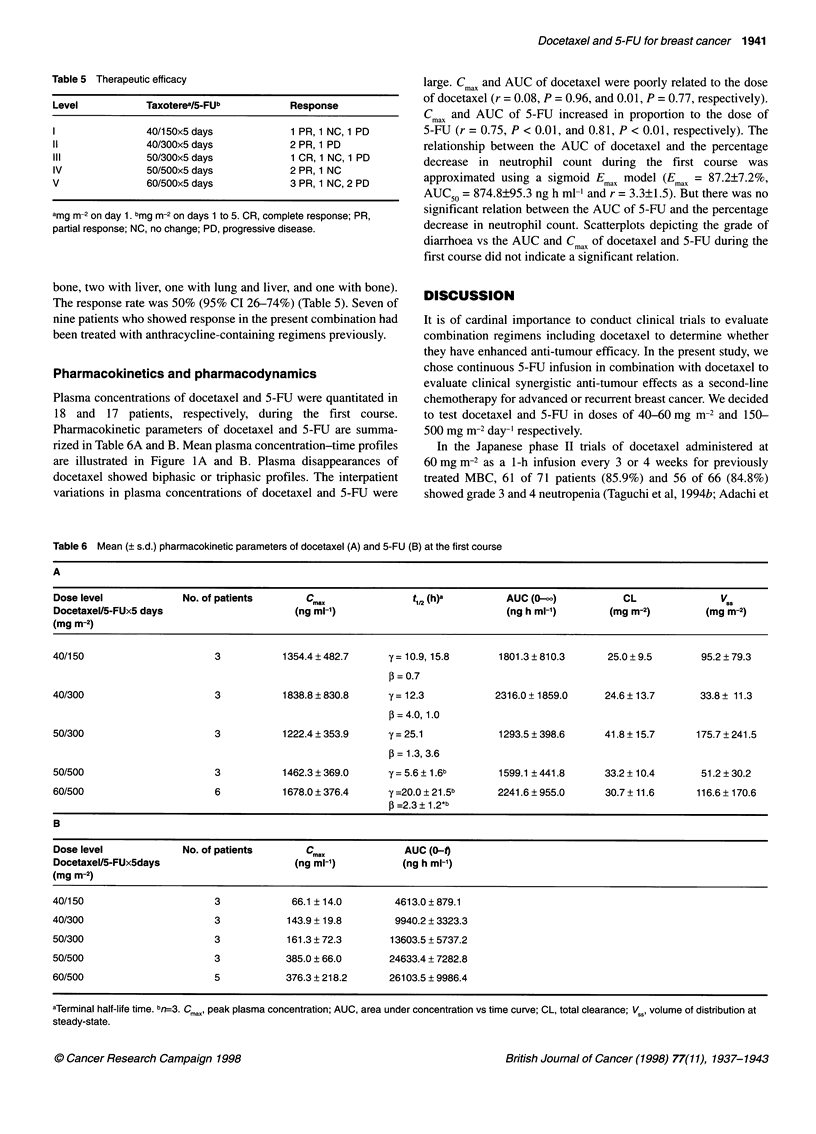
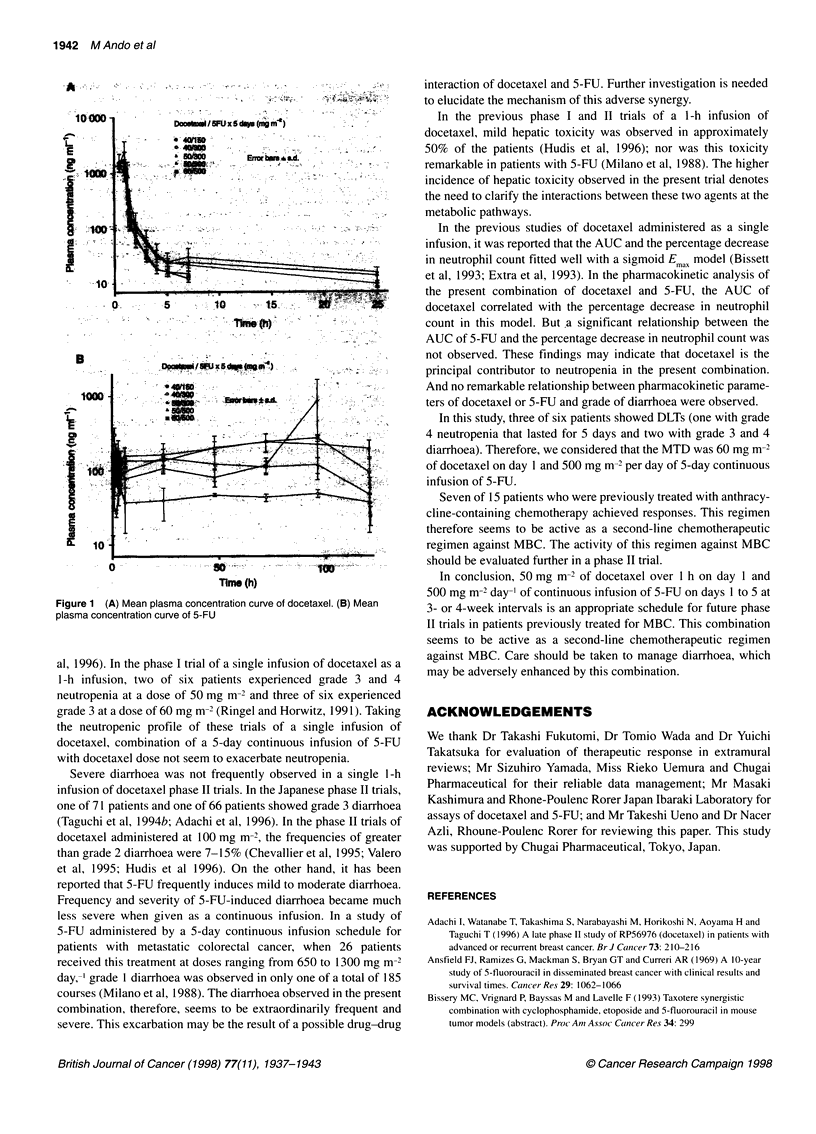
To determine the maximum-tolerated doses (MTDs), the dose-limiting toxicities (DLTs) and the recommended doses for further trials of docetaxel in combination with a 5-day continuous infusion of 5-fluorouracil (5-FU) in advanced or recurrent breast cancer patients who had been treated previously with at least one chemotherapeutic regimen, patients were treated with docetaxel as a 1-h infusion on day 1 followed by 5-FU as a continuous infusion on days 1 through 5 every 3-4 weeks. Three or six patients were assessed at the following escalating dose levels of docetaxel/5-FU per day: 40/150, 40/300, 50/300, 50/500 and 60/500 mg m(-2). Nineteen patients entered this trial, of whom 18 could be assessed for adverse event and therapeutic efficacy. The DLTs were neutropenia and diarrhoea. The MTDs were 60 mg m(-2) of docetaxel on day 1 and 500 mg m(-2) per day of 5-day continuous infusion of 5-FU. One of 18 patients achieved a complete response and eight achieved partial response (over all response rate: 50%). The recommended doses of docetaxel and 5-day continuous infusion of 5-FU for a phase II trial are 50 mg m(-2) and 500 mg m(-2) per day every 3 or 4 weeks.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi I., Watanabe T., Takashima S., Narabayashi M., Horikoshi N., Aoyama H., Taguchi T. A late phase II study of RP56976 (docetaxel) in patients with advanced or recurrent breast cancer. Br J Cancer. 1996 Jan;73(2):210–216. doi: 10.1038/bjc.1996.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansfield F. J., Ramirez G., Mackman S., Bryan G. T., Curreri A. R. A ten-year study of 5-flurouracil in disseminated breast cancer with clinical results and survival times.. Cancer Res. 1969 May;29(5):1062–1066. [PubMed] [Google Scholar]
- Bissett D., Setanoians A., Cassidy J., Graham M. A., Chadwick G. A., Wilson P., Auzannet V., Le Bail N., Kaye S. B., Kerr D. J. Phase I and pharmacokinetic study of taxotere (RP 56976) administered as a 24-hour infusion. Cancer Res. 1993 Feb 1;53(3):523–527. [PubMed] [Google Scholar]
- Cameron D. A., Gabra H., Leonard R. C. Continuous 5-fluorouracil in the treatment of breast cancer. Br J Cancer. 1994 Jul;70(1):120–124. doi: 10.1038/bjc.1994.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier B., Fumoleau P., Kerbrat P., Dieras V., Roche H., Krakowski I., Azli N., Bayssas M., Lentz M. A., Van Glabbeke M. Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: a phase II trial of the Clinical Screening Cooperative Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol. 1995 Feb;13(2):314–322. doi: 10.1200/JCO.1995.13.2.314. [DOI] [PubMed] [Google Scholar]
- Extra J. M., Rousseau F., Bruno R., Clavel M., Le Bail N., Marty M. Phase I and pharmacokinetic study of Taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res. 1993 Mar 1;53(5):1037–1042. [PubMed] [Google Scholar]
- Gregory W. M., Smith P., Richards M. A., Twelves C. J., Knight R. K., Rubens R. D. Chemotherapy of advanced breast cancer: outcome and prognostic factors. Br J Cancer. 1993 Nov;68(5):988–995. doi: 10.1038/bjc.1993.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford N. H., Sheiner L. B. Kinetics of pharmacologic response. Pharmacol Ther. 1982;16(2):143–166. doi: 10.1016/0163-7258(82)90051-1. [DOI] [PubMed] [Google Scholar]
- Hudis C. A., Seidman A. D., Crown J. P., Balmaceda C., Freilich R., Gilewski T. A., Hakes T. B., Currie V., Lebwohl D. E., Baselga J. Phase II and pharmacologic study of docetaxel as initial chemotherapy for metastatic breast cancer. J Clin Oncol. 1996 Jan;14(1):58–65. doi: 10.1200/JCO.1996.14.1.58. [DOI] [PubMed] [Google Scholar]
- Jabboury K., Holmes F. A., Hortobagyi G. 5-Fluorouracil rechallenge by protracted infusion in refractory breast cancer. Cancer. 1989 Aug 15;64(4):793–797. doi: 10.1002/1097-0142(19890815)64:4<793::aid-cncr2820640404>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Jäger W., Czejka M. J., Schüller J., Fogl U., Czejka E., Lackner H. Rapid and simple high-performance liquid chromatographic assay for 5'-fluorouracil in plasma for bioavailability studies. J Chromatogr. 1990 Nov 16;532(2):411–417. doi: 10.1016/s0378-4347(00)83792-5. [DOI] [PubMed] [Google Scholar]
- Lavelle F., Bissery M. C., Combeau C., Riou J. F., Vrignaud P., André S. Preclinical evaluation of docetaxel (Taxotere). Semin Oncol. 1995 Apr;22(2 Suppl 4):3–16. [PubMed] [Google Scholar]
- Milano G., Roman P., Khater R., Frenay M., Renee N., Namer M. Dose versus pharmacokinetics for predicting tolerance to 5-day continuous infusion of 5-FU. Int J Cancer. 1988 Apr 15;41(4):537–541. doi: 10.1002/ijc.2910410411. [DOI] [PubMed] [Google Scholar]
- Ravdin P. M., Burris H. A., 3rd, Cook G., Eisenberg P., Kane M., Bierman W. A., Mortimer J., Genevois E., Bellet R. E. Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedione-resistant breast cancer. J Clin Oncol. 1995 Dec;13(12):2879–2885. doi: 10.1200/JCO.1995.13.12.2879. [DOI] [PubMed] [Google Scholar]
- Ringel I., Horwitz S. B. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991 Feb 20;83(4):288–291. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- Taguchi T., Furue H., Niitani H., Ishitani K., Kanamaru R., Hasegawa K., Ariyoshi Y., Noda K., Furuse K., Fukuoka M. [Phase I clinical trial of RP 56976 (docetaxel) a new anticancer drug]. Gan To Kagaku Ryoho. 1994 Sep;21(12):1997–2005. [PubMed] [Google Scholar]
- Taguchi T., Mori S., Abe R., Hasegawa K., Morishita Y., Tabei T., Sasaki Y., Fujita M., Enomoto K., Hamano K. [Late phase II clinical study of RP56976 (docetaxel) in patients with advanced/recurrent breast cancer]. Gan To Kagaku Ryoho. 1994 Nov;21(15):2625–2632. [PubMed] [Google Scholar]
- Tobinai K., Kohno A., Shimada Y., Watanabe T., Tamura T., Takeyama K., Narabayashi M., Fukutomi T., Kondo H., Shimoyama M. Toxicity grading criteria of the Japan Clinical Oncology Group. The Clinical Trial Review Committee of the Japan Clinical Oncology Group. Jpn J Clin Oncol. 1993 Aug;23(4):250–257. [PubMed] [Google Scholar]
- Valero V., Holmes F. A., Walters R. S., Theriault R. L., Esparza L., Fraschini G., Fonseca G. A., Bellet R. E., Buzdar A. U., Hortobagyi G. N. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol. 1995 Dec;13(12):2886–2894. doi: 10.1200/JCO.1995.13.12.2886. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]


