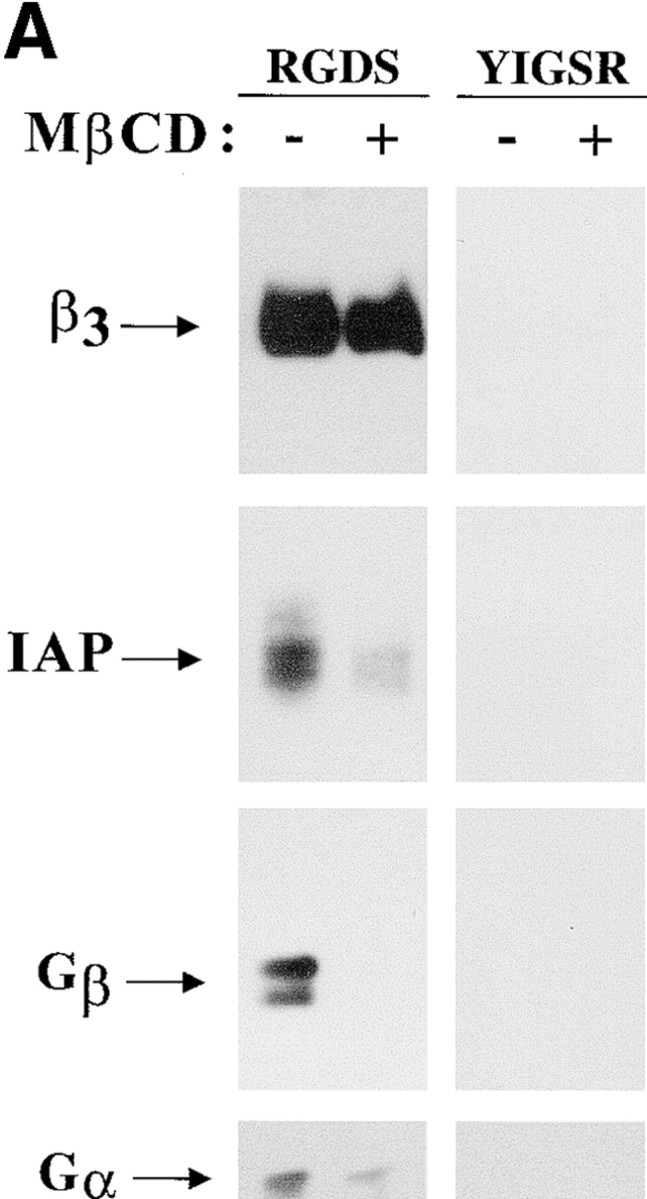
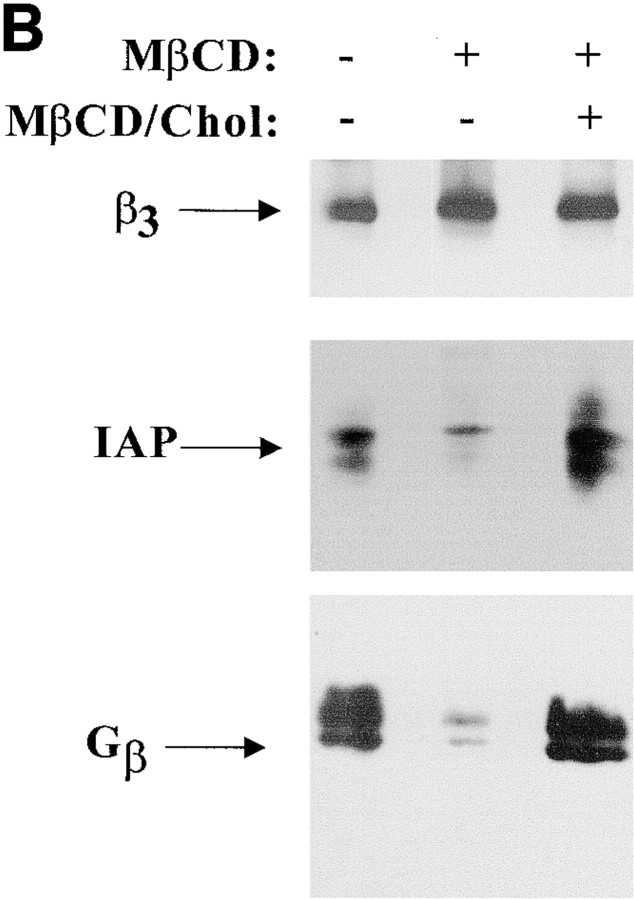
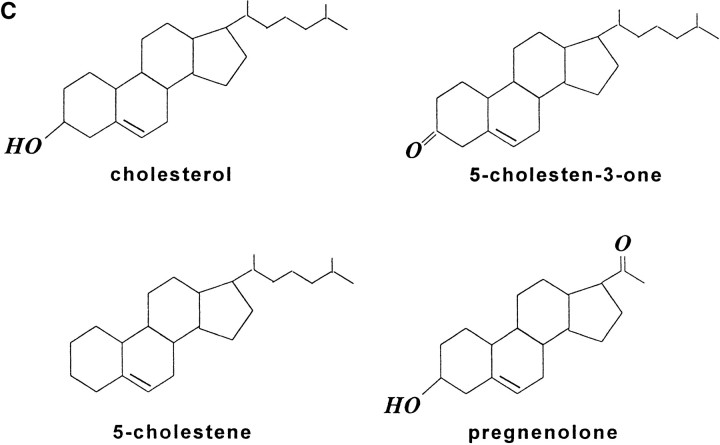
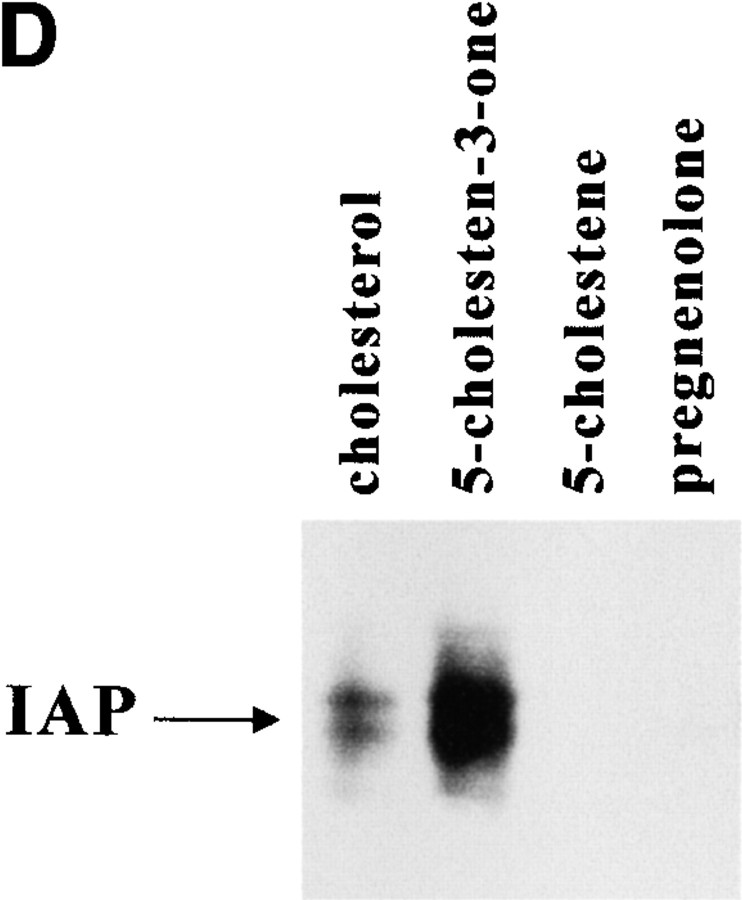
Figure 1.
Cholesterol is in IAP/αvβ3/G protein complexes. A, Complexes of αvβ3, IAP, and G proteins from αvβ3- and IAP-expressing OV10 cells were isolated using beads coated with the αvβ3 ligand, RGDS, as described in Materials and Methods, then treated in vitro with buffer or 1 mM MβCD, and finally analyzed by SDS-PAGE and Western blotting using the β3 specific mAb 7G2, the IAP specific mAb B6H12, or rabbit antibodies specific for Gα or Gβ. Identical results were obtained with complexes obtained with Vn-coated beads or with anti-αvβ3–coated beads (data not shown). No protein reactive with any of the detecting antibodies was obtained using beads coated with the cell binding laminin peptide, YIGSR (Iwamoto et al. 1987) that does not bind integrins (Graf et al. 1987b). B, Coimmunoprecipitations were performed using beads coated with the β3 specific mAb 1A2 from buffer treated (lane 1), cholesterol-depleted (lane 2), or cholesterol-repleted (lane 3) OV10 cells expressing αvβ3 and IAP. Cholesterol depletion with MβCD and repletion with MβCD-cholesterol complexes were performed as described in Materials and Methods. No protein was immunoprecipitated using the irrelevant mAb 543 (data not shown). C, Structures of the cholesterol analogues used for preparation of the MβCD inclusion complexes. D, Coimmunoprecipitations were performed using beads coated with the β3 specific mAb 1A2 from cholesterol-depleted cells repleted with cholesterol or one of the analogues, 5-cholestene-3-one, 5-cholestene, or pregnenolone. Western blotting for IAP is shown. The amount of αvβ3 immunoprecipitated was similar for each sample, as determined by Western blotting (data not shown). Each experiment is representative of at least three repetitions.