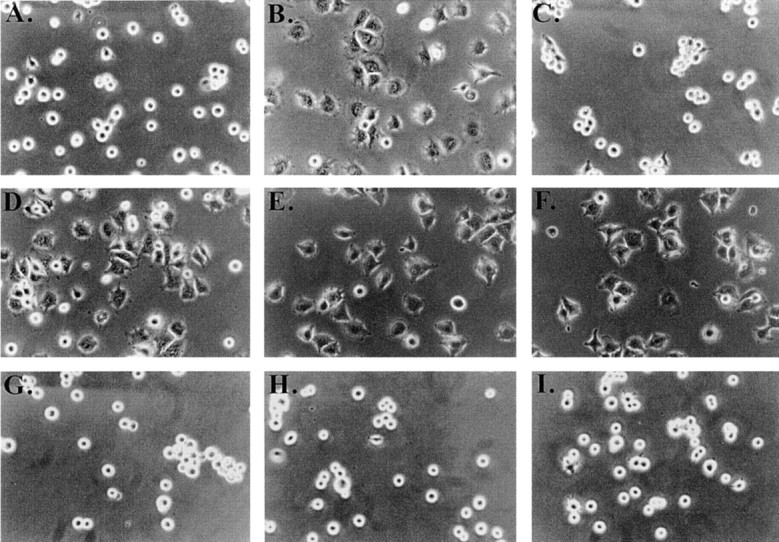
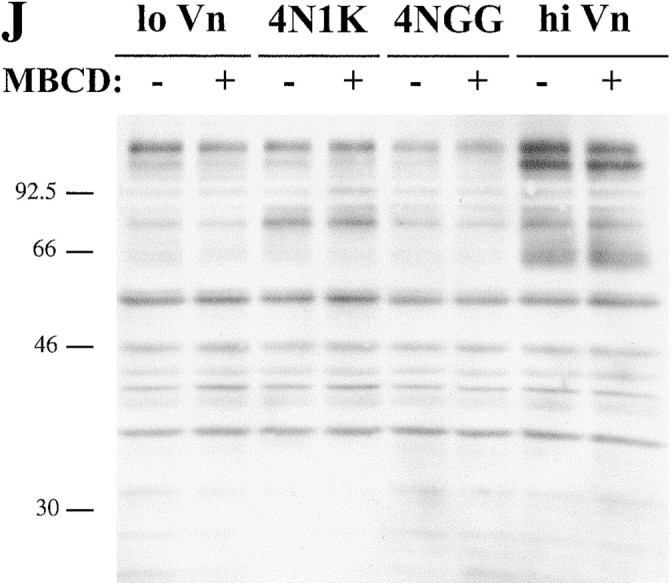
Figure 2.
Effect of cholesterol depletion on spreading and tyrosine phosphorylation of C32 melanoma cells. (A–D, G–I) C32 cells were allowed to spread on plates coated with a suboptimal amount of Vn (0.125 μg/ml) for 30 min at 37°C in the absence (A) or presence (B–D, G–I) of 20 μM TSP peptide 4N1K in solution. C, Cells were pretreated with 10 mM MβCD for 15 min to remove plasma membrane cholesterol. D, Cholesterol–MβCD inclusion complexes were added to cholesterol-depleted cells to replenish plasma membrane cholesterol. E and F, Cells spreading on plates coated with 1 μg/ml Vn in the absence (E) or presence (F) of 10 mM MβCD. No 4N1K peptide was added. Spreading was complete within 30 min at 37°C, when pictures were taken. G–I, Cholesterol-depleted cells were treated with 4N1K peptide and MβCD-inclusion complexes made with the cholesterol analogues 5-cholestene-3-one (G), 5-cholestene (H), and pregnenolone (I). 5-cholestene-3-one inhibited C32 spreading on high (1 μg/ml) Vn, demonstrating a nonspecific inhibition of cell spreading, but the other two analogues had no such effect (data not shown). J, C32 cells, with or without treatment with 10 mM MβCD, were incubated on low density Vn (0.125 μg/ml) in the absence or presence of 20 μM 4N1K or the scrambled control peptide 4NGG (Gao et al. 1996b). Alternatively, cells were allowed to spread on high concentrations of Vn (1 μg/ml). After cell lysis and SDS-PAGE, Western blotting was performed using antiphosphotyrosine mAb 4G10.