Figure 8.
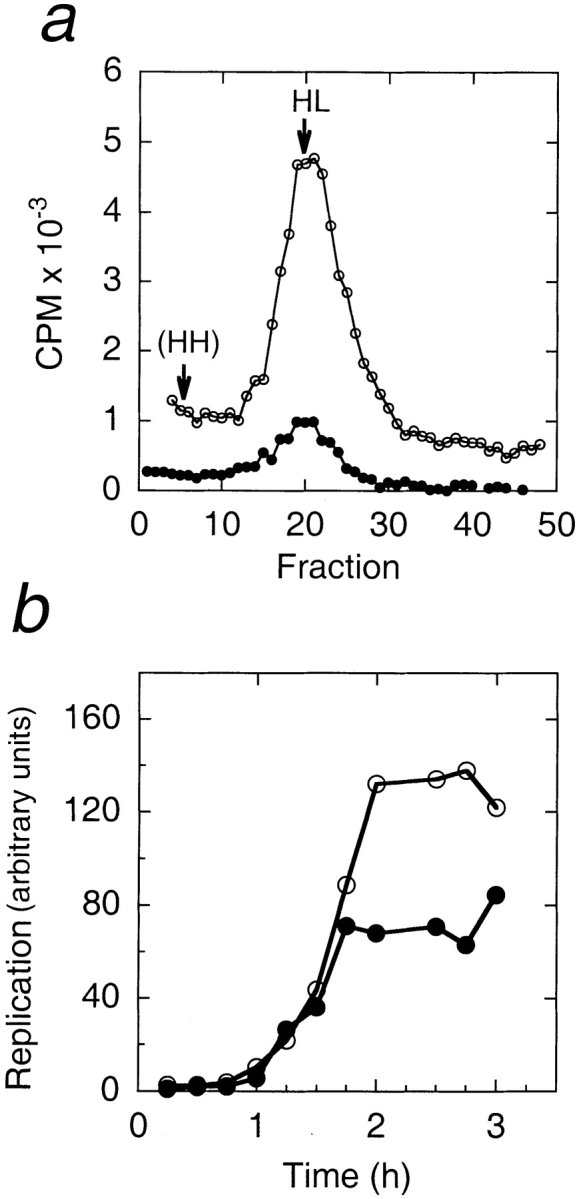
BrdU density substitution experiment, and replication time course. (a) Density substitution experiment. Nuclei were assembled for 2 h in reactions containing BrdU and α[32P]dCTP, in the presence or absence of 3.3 μM fragment 1–408. The substituted DNA was purified and separated by centrifugation on equilibrium CsCl gradients (see Materials and Methods). Replicated DNA (cpm of incorporated α[32P]dCTP) in each gradient fraction is plotted against gradient fractions, which had equal densities as determined by refractive index measurements. HL indicates the density of heavy–light DNA (1.746 g/ml), and HH the expected density of heavy–heavy DNA (1.80 g/ml; Walter et al., 1998). Filled circles indicate reactions lacking LAP2; open circles indicate reactions containing 3.3 μM LAP2 fragment 1–408. (b) Replication time course. Nuclei were assembled in reactions containing α[32P]dCTP, in the presence or absence of fragment 1–408. Aliquots were removed every 15 min for 3 h, and processed by running purified DNA on agarose gel, and quantitating the incorporated [32P]dCTP by PhosphorImager (see Materials and Methods). Filled circles indicate reactions lacking LAP2; open circles indicate reactions containing 3.3 μM LAP2 fragment 1–408.
