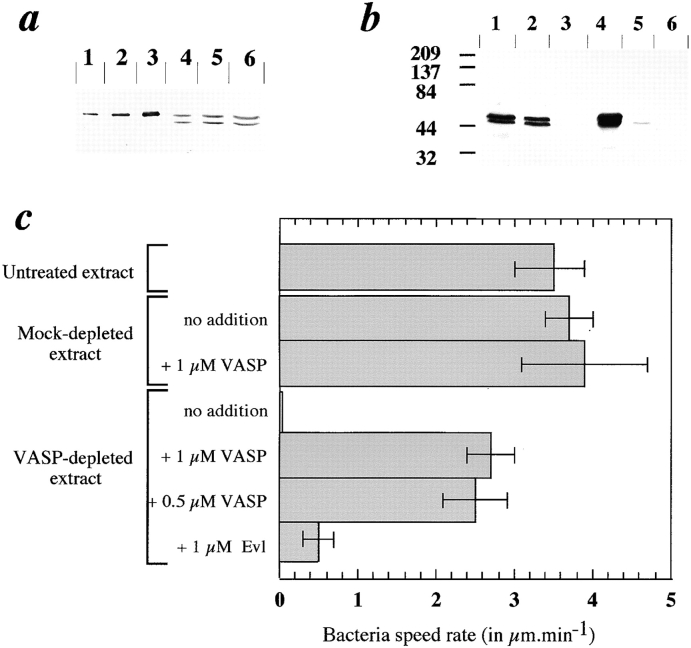
Figure 2.
Immunodepletion of VASP from platelet extracts and Listeria movement in VASP-depleted extracts supplemented with recombinant VASP or Evl. (a) Quantitation of VASP in platelet extracts. 0.5 (lane 1), 1 (lane 2), and 2 pmol (lane 3) of recombinant VASP and 0.66 (lane 4), 1.33 (lane 5), and 2.66 μl (lane 6) of platelet extract were electrophoresed and immunoblotted using anti-VASP (M4 polyclonal antibody). Comparison of the densitometric patterns of the standards and of platelet VASP using the NIH Image analysis software led to an estimate of 0.7 μM VASP in the extracts. (b) Analysis of the immunodepletion of VASP from platelet extracts. Lane 1, whole extract; lane 2, mock-depleted extract; lane 3, VASP-depleted extract (two cycles of depletion using Dynabeads); lane 4, Dynabead-bound VASP after the first cycle of depletion; lane 5, Dynabead-bound VASP after the second cycle of depletion; and lane 6, control beads (not coated with anti-VASP) used for the mock depletion. The Western blot was revealed using M4 polyclonal anti-VASP. (c) Mean rates of movement of Listeria in VASP-depleted extracts and in depleted extracts supplemented with VASP or Evl recombinant proteins. 10–15 rate measurements were performed for each sample. At least three independent experiments were carried out.