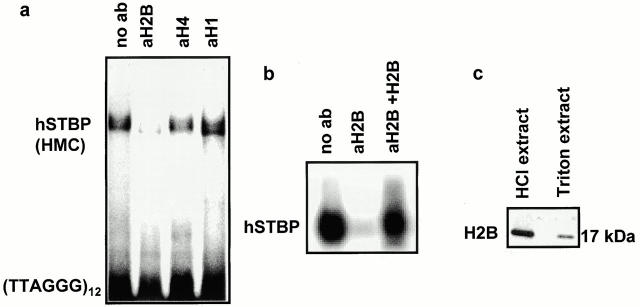
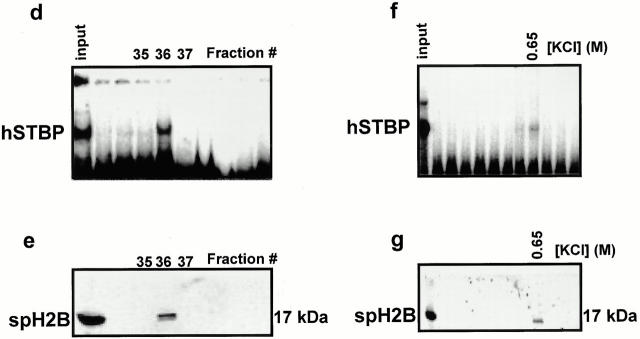
Figure 3.
Sperm-specific variant of human histone H2B is a component of hSTBPTR. (a) Antibodies against H2B, but not against other histone fractions suppress formation of hSTBP-[TTAGGG]12 complexes. Telomere binding activity was determined in gel-shift assay in the presence of the indicated antibodies. (b) Formation of the hSTBP-[TTAGGG]12 complex was restored after preincubation of anti–H2B serum with the purified HeLa H2B. (c) Western analysis of acid-soluble and Triton X-100–extracted proteins of human sperm. (d–g) Partial purification of hSTBPTR shows that spH2B histone coelutes with telomere-binding activity. Gel filtration: eluted fractions were assayed for telomere-binding activity (d), and Western blotting using anti–H2B antibodies (e). Ion-exchange chromatography: fractions were eluted by linear gradient of KCl, assayed for binding to [TTAGGG]12 probe (f) and in Western blotting (g).